About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Engineering
The method of survived slices of native tissue is currently widely used in cell biology. The method has found a particularly extensive application in studies of the central nervous system (Alger et al. 1982; Bak et al. 1980; Brain Slices, 1982; O'Brien, McCully, 1981; Budantsev, 2015; Levin, Godukhin, 2011; Savina et al., 2013; Schurr et al. 1984, 1987; etc.). However, this method can also be applied in other fields of experimental cell biology, e. g., in combination with electron microscopy, long-term cultivation of tissue explants, in intravital microscopy, etc.
Slices of native tissue are prepared using special microtomes of two types: choppers and vibroslicers. Commercial devices manufactured by some companies have a number of advantages and disadvantages. Therefore, the development of novel technical solutions of native tissue microtomes of these types is being continued.
On the basis of a rotary microtome previously developed chopper for sections of native tissue of animals and plants (Budantsev, 2015).
This article describes the design of a microtome sectioning native tissue for cell physiology and microscopy. The base of the microtome is developed on the scheme of the rotary microtome.
Results
1. General scheme of the rotary microtome
The construction of a microtome is based on the standard scheme of a rotary microtome for the preparation of histological slices of a fixed tissue (Fig. 1).
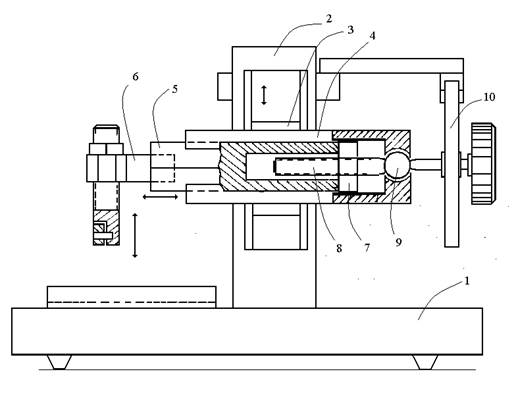
Fig. 1. Scheme of the main microtome unit (rotary microtome). See text.
The microtome unit and an electrical drive (not shown in the scheme) are mounted on the general base (1). The electrical drive is operated by a foot pedal.
The microtome unit consists of a vertical frame (2) on which a carriage (3) in wedge slide guides performs motor-driven vertical reciprocating motions. On the carriage, a plate (4) with wedge slide guide is mounted. A prism (5) with a tissue holder (6) fixed on the front face moves along the plate (4). On the other end of the prism, there is a nut (7) in which a special microscrew (8) moves. On the back end of the microscrew axis, there is a spherical bearing (9) and a ratchet wheel (10). In each cycle of the vertical motion of the carriage, the microscrew moves the prism forward for a distance set by the ratchet wheel. In a standard rotary microtome, the microscrew has a fine left-hand thread. A tissue sample sealed with paraffin is fixed on the prism. The microtome knife is fastened on the base of the microtome.
2. The microtome for native tissues
Figure 2 shows the scheme of microtome for native tissues with a left-hand pentathread microscrew.
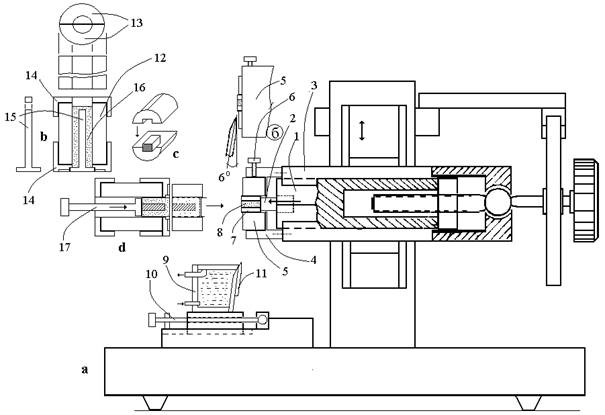
Fig. 2. The scheme of Microtome for native tissue. See text.
At the front edge of prism (1), a pusher (2) is fixed. On plate (3), a cylindrical casing is fixed into the front end of which a ring plate (5) is inserted. The ring plate is fixed in position by a screw (6). A cylindrical agar tray (7) with a tissue sample (8) is fitted into the central channel of the ring plate. As the prism moves forward, the pusher pulls the agar tray out of the ring plate channel over a preset distance. At the beginning of the job, the pusher is set by a handle of the microscrew to the terminal back position. As the microtome unit moves down, the outward-projecting part of the cylindrical agar tray with a tissue sample is cut by a razor blade and thrown into a cuvette located at the base of the chopper. The position of the cuvette relative to the ring plate is fixed by the screw (10). The front wall of the cuvette has an angle equal to the honing angle of the razor blade (about 6o). The razor blade (11) is fixed on the front wall of the cuvette using a clamp plate with a thin silicon gasket. The plane of the front face of the cutting edge slides on the front plane of the ring plate, and the tissue section together with the agar tray falls into the cuvette (9) with physiological solution.
For the preparation of a cylindrical agar tray and loading of a tissue sample into the tray and ring plate, a special device was developed (Fig. 3). The device consists of a hollow cylinder (13) divided into two halves (14). The cylinder is fixed between two abutments (15). An end cup (16) is inserted into the central part of the hollow cylinder, and the internal space of the cylinder is filled with molten agar (17). After the stiffening of agar, the device is taken apart. Without being taken from the hollow cylinder, the agar tray is cut lengthwise into two halves by a razor blade (the blade is inserted into a slit between two halves of the hollow cylinder). Then the tissue sample is put into the ring plate of the chopper. To do this: (1) a tissue sample is placed into the agar tray (Fig. 3d); (2) the agar tray with the tissue is placed inside the cylinder, and the device is assembled (Fig. 3e); (3) the assembled device is put against the ring plate of the chopper, and the agar tray with the tissue is pushed into the channel of the ring plate up to the stop to the pusher.
Discussion
A number of devices for the manual and technical preparation of sections of native tissues have been developed (see review in Budantsev, 2015)
It was found that the optimal thickness of a section of native tissue for electrophysiological and metabolic studies lies in the range of 250-350 μm. At this thickness, a good relationship between destroyed and intact cells and an adequate supply of the inner part of a slice with oxygen in running water are provided (Alger et al. 1982, etc.).
Using the microtome it is possible to prepare slices of animal and plant tissues of 100-400 μm thick.
The above-described microtome may be used for preparation of native tissues slices in cell physiology and microscopy.
2. Bak J.J., Misgeld U., Weiler M., Morgan E. (1980) The preservation of nerve cells in rat neostriatal slices maintained in vitro. A morphological study. Brain Res. 197: 341-353.
3. Brain slices, Dingledine R. (Ed.) New York, 1982. 350 p.
4. O’Brien T.P., McCully M.E. (1981) The study of plant structure principles and selected methods, Termarcarphi Pty.Ltd., Melburn, Australia. pp.4.2-4.7.
5. Budantsev A.Yu. (2015) Modification of the rotary microtome for native tissue, Intern. J. of applied and fundamental investigations (rus.), №6 (part 1)
6. Levin S.G. and Godukhin O.V. (2011) Anti-inflammatory cytokines, TGF-β1 and IL-10, exert anti-hypoxic action and abolish posthypoxic hyperexcitability in hippocampal slice neurons: comparative aspects. Experimental Neurology, USA: Elsevier., 232(2), 329-332.
7. Savina T.A., Shchipakina T.G., Levin S.G., Godukhin O.V. (2013) Interleukin-10 prevents the hypoxia-induced decreases in expressions of AMPA receptor subunit GluA1 and alpha subunit of Ca2+/calmodulin-dependent protein kinase II in hippocampal neurons. Neuroscience Letters, 534, 279-284
8. Schurr F., Reid K.H., Tseng M.T., Endmondsards H.L. (1984) The stability of the hippocampal slices preparation: an electrophysiological and ultrastrtuctural analysis. Brain Res. 297, 357-362.
9. Schurr F. et al. (Eds.) Brain Slices. Fundamentals, applications and implication. New York, 1987
Budantsev A. Yu. THE MICROTOME FOR NATIVE TISSUES. International Journal Of Applied And Fundamental Research. – 2015. – № 1 –
URL: www.science-sd.com/460-24768 (05.03.2026).









 PDF
PDF