About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Medical sciences
Material and methods.
We studied the action of water solutions of DNIC on whole blood of healthy peoples. Different volumes of DNIC solutions (substance concentration – 3 mmol/l; used volume – 0,05; 0,1 and 0,2 ml.) were injected in blood samples (5 ml.) DNIC synthesis was executed with A.F. Vanin’s method (2005) [5]. DNIC level in solution was estimated by spectrometry on 310 and 360 nm. Exposition time was 3 min. Control blood sample was intact. We studied pH, gases partial pressure, main ions level and parameters of acid-base balance in blood plasma of all samples with automatic analyzer ABL-77. Statistic processing of the data was accomplished by the programs Microsoft Excel 2003 and Statistica 6.0. The descriptive statistics data is shown in the article.
Results.
It was stated, that processing of whole blood with DNIC leads to dose dependent increasing of pH (fig. 1). So, injection of low dose of DNIC (0,15 mcg) caused small elevation of this parameter (in 0,08 un.; p<0,1). Use of higher doses of NO stipulates dose-dependent elevation of blood pH. Maximal NO dose (0,6 mcg) increases this parameter on 0,143 un. (p<0,05).
Our experiments shown, that introduction of DNIC water solutions in biological fluid results in the changes of partial pressure of oxygen and carbon dioxide (fig. 2). In particular, all investigated doses of nitric oxide leads to proportional decreasing of partial pressure of carbon dioxide, but this effect is not associated with NO dose clearly.
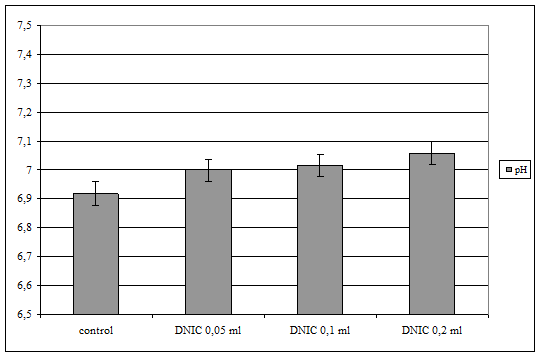
Fig. 1. pH level of blood samples under injection of different doses of dinitrozyl iron complexes (DNIC)
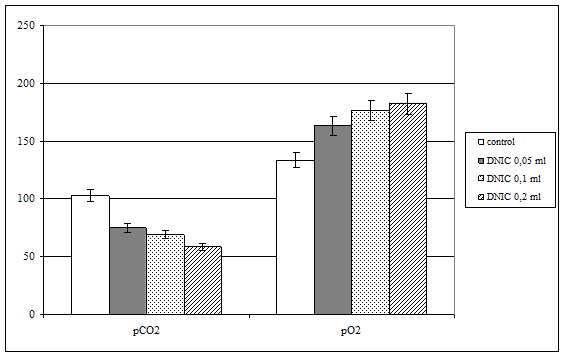
Fig. 2. Blood partial pressure of oxygen and carbon dioxide under injection of different doses of dinitrozyl iron complexes (DNIC)
In addition, we fixed elevation of oxygen partial pressure, which dynamics was correlates directly with quantity of injected DNIC (r=+0,88; p<0,01). On the base of use of DNIC water solution, but not NO-containing gas flow we supposed, that registered positive changes of estimated parameters is result of blood oxygen capacity.
Conclusion
Our experiments allow to state marked action of DNIC water solutions of physical and chemical properties of human blood in vitro. This effect includes moderate blood alkalization and optimization of gases balance as a result of decreasing of carbon dioxide partial pressure with elevation this parameter for oxygen. So, these data indicate on positive action of DNIC on estimated blood indexes.
2. Mikoyan V.D., Serezhenkov V.A., Brazhnikova N.V. et al. Formation of paramagnetic nitrosyl complexes of nonheme iron in the animal organism with the participation of nitric oxide from exogenous and endogenous sources // Biophysics. 2004. Vol. 41. P. 110-116.
3. Surio Rahmanto Y., Kalinowski D.S., Lane D.J. et al. Nitrogen monoxide (NO) storage and transport by dinitrosyl-dithiol-iron complexes: long-lived NO that is trafficked by interacting proteins // J. Biol. Chem. 2012. Vol. 287. P. 6960-6968.
4. Vanin A.F. Dinitrosyl iron complexes with thiolate ligands: physico-chemistry, biochemistry and physiology // Nitric oxide Biol. Chem. 2009. Vol. 21. P. 1-13.
5. Vanin A.F., Lozinskiy V.I., Kapelko V.I. Polymer composition for formation of stable form of dinitrozyl iron complexes and method of its synthesis. Patent of Russian Federation №2291880. 2005.
Martusevich A.K., Soloveva A.G., Peretyagin S.P., Vanin A.F. Action of dinitrozyl iron complexes on some physical and chemical parameters of human blood in vitro. International Journal Of Applied And Fundamental Research. – 2014. – № 1 –
URL: www.science-sd.com/456-24496 (19.01.2026).









 PDF
PDF