About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
The liquid industrial chloroparaffins are usually used, as the second plasticizers in combination with the primary ones, for example, the phthalate plasticizers, because of their limited plasticizing capacity and the compatibility with the polymers. The chlorinated paraffins are usually given the polymers products a number of the valuable properties, such as the increased fire resistance, the cold resistance, the mechanical strength, the resistance to the hydrocarbons, the fats and the oils action. In addition, the chloroparaffins are quite non – toxic (e.g. the hazard class 4) [1], in contrast to the phthalate plasticizers (e.g. the hazard class 2) [2], are usually distinguished by their low cost and the raw materials availability for their further production.
It has been found by us [3], that in composed of the final products of the chlorinated paraffins liquid – phase oxidation by oxygen, the higher chlorinated carboxylic acids (HCCA) are produced. The HCCA solutions in the chloroparaffin are the new promising raw material for the plasticizing [4] and the stabilizing compositions [5] creation.
The higher thermal paraffins oxidation challenge, more individual long chain paraffinic hydrocarbons (e.g. the decane, the hexadecane and etc.) by oxygen or by air has been dealt in a number of several papers. The conversion scheme and the products composition content of the paraffinic hydrocarbons oxidation have been investigated in detail, and moreover, the mathematical dependences and their relationships, having allowed to be controlled over the whole process, have already been developed thoroughly. So, it, moreover, has been shown, that the acids are the final products, and, in some studies, the complex esters are also referred, as the oxidation end products [6 – 19].
The higher chlorinated hydrocarbons oxidation has already been described very little [20 – 22]. Probably, the chlorinated hydrocarbons oxidation is practically subjected to the basic laws and their regulations of the chain – radical hydrocarbons oxidation process by the molecular oxidation, however, the chlorine presence in the molecule is conditioned and determined the conversions peculiarities characteristics of the chlorinated long – chain hydrocarbons.
It should be noted, that the chlorinated hydrocarbons are practically more resistant to the oxidation, than their non – chlorinated analogues. In particular, the industrial chloroparaffins, having presented themselves the long – chain chlorinated hydrocarbons mixture with the chlorine atom different number and its position in the molecule, are not practically subjected to the thermal oxidation.
Thus, it is well – known, that the metal compounds of the variable valence are the hydrocarbons oxidation catalysts [23]. So, by analogy with the paraffins oxidation process, in order to be obtained the synthetic fatty acids [6], for the chloroparaffins oxidation, the manganese and the potassium salts had already been investigated, as the catalyst, which was enabled us to be carried out the chloroparaffins oxidation [3] by air.
So, the chloroparaffins are practically received, and they are prepared by the paraffins chlorination without the chlorinated organic products obtained separation, that is why they are the complex multi – component mixtures of the non – permanent content. This, first of all, is made it quite difficult to be studied the kinetics and also the mechanism of the chlorinated paraffins oxidation process.
For the management of the chlorinated paraffins oxidation process in this work, we have already developed the mathematical dependencies and their relationships, having allowed to be carried out the chlorinated paraffins oxidation by air in the presence of the manganese salts up to the certain value of the acid number.
The liquid industrial chloroparaffins are being produced by the following brands: CHP-250, CHP-30, CHP-470, CHP-52, which are differed in the combined chlorine content. One fraction of the oil paraffin is practically used with the chain length С14-С17 for the chlorinated paraffins production of these above – indicated brands. In this connection, the chain length of the hydrocarbon radical in the modeling process cannot be considered, as the experiment factor.
The Table No.1. The Physical Properties of the Liquid Industrial Chlorinated Paraffins [1]
|
The Properties |
The Liquid Chloroparaffins |
||||
|
СHР-250 |
CHP-30 brand А |
CHP-30 brand В |
CHP-470 |
CHP-52 |
|
|
1 |
2 |
3 |
4 |
5 |
6 |
|
The mass fraction of the fixed chlorine, % |
24-29 |
28-32 |
28-32 |
45-49 |
50-54 |
|
The mass fraction of the acids in terms of НСl, %, no more |
0,004 |
0,004 |
0,004 |
0,005 |
0,005 |
|
The density at 25ºС, g/cm3 within |
- |
0,98-1,02 |
0,98-1,02 |
- |
1,25-1,26 |
|
The density at 20ºС, kg/m3 within |
960-1020 |
- |
- |
1185-1235 |
- |
|
The thermo stability based on the cleaved HCl, %, no more |
- |
0,20 |
0,20 |
0,20 |
0,15 |
|
The viscosity, P at 25 ºС, within |
- |
- |
- |
- |
10-16 |
|
The mass fraction of the iron ions, %, no more |
0,002 |
0,004 |
0,004 |
0,004 |
0,006 |
|
The color by the iodine scale, mg J2/100 cm3, no more |
- |
- |
- |
4 |
- |
|
The color by the optical density, no more |
- |
- |
0,35 |
- |
0,60 |
The Experimental Part
The CHP-250, CHP-30, CHP-470, CHP-52 brands chloroparaffins have been used, as the substrate (see the Table No. 1).
The catalyst is presented itself the potassium, water, the acetic acid and the stearic acid permanganate mixture. So, the manganese salts of the corresponding acids are usually being formed directly in the reaction mixture during the chlorinated paraffins oxidation.
Thus, the oxidation has been conducted in the glass reactor of the bubbling type values column height to the diameter 10:1, the 200 ml volume. The air has been fed through the sparger (e.g. the air flow rate 6 l/(min·kg)), it has been experimentally established, that, under these conditions, the oxidation is being proceeded in the kinetic region, and diffusion inhibition can be neglected. The temperature has been maintained the first 2 hours at 120 0С for the catalyst complex formation, then the oxidation process has been carried out at 107-1080С. The reactor temperature control has been conducted by the heat – carrier (e.g. glycerol) circulation through the reactor jacket.
The oxidation process passing control has been carried out on the acids content (e.g. the acid number change (e.g. AN, mg КОН/ g) over the time, having allowed to be determined the carboxyl groups concentration in the oxidation products). The IR-, NMR-spectroscopy, chromatography mass – spectroscopy have already been used for the further additional oxidation products identification.
The Results’ Discussion
The experiments’ results on the chlorinated paraffins oxidation, having selected grades, are shown in Figure 1:
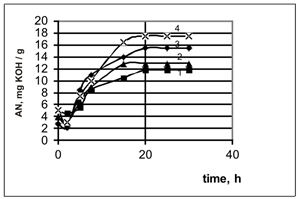
The Figure 1. The chlorinated paraffins oxidation of the different brands under the same conditions
(The air flow rate 6 l/(min·kg). The catalyst content is 8% (wt.). The temperature is 107 – 108°С).
1- ■ – СHP-250, 2- ▲ – CHP-30, 3-¨- CHP-470, 4- х– CHP-52.
It has been found, that the chloroparaffins with the chlorination varying degrees (e.g. CHP-250, CHP-30, CHP-470, CHP-52) are being given the acid number various values, under the same conditions of the oxidation process (e.g. the time of 20 hours, the temperature of 107-1080С, the air flow rate 6 l/( min·kg)).
The special experiments have been conducted in the chlorinated paraffins oxidation in the presence of the manganese acetate and the stearate (e.g. catalyst) mixture, for the accumulation rate dependence investigation of the acid number (AN) of the catalyst concentration.
It has been found in the univariate experiment for the CHP-30 brand chloroparaffin, that the concentration changing of the catalyst from 4 up to 8% (e.g. by its weight) is practically resulted in the high rate of the acid number (AN) accumulation.
Thus, it has been found, that the catalyst concentration and the hydrocarbon chlorination degree may be the control parameters for the chlorinated paraffins oxidation process.
So, in this connection, it was interesting to be established the mathematical dependence and its relationship, having resulted in the acid number (AN) of the initial parameters: the raw materials chlorination (nCl) and the catalyst concentration (CKt) degrees:
![]()
So, the accumulation average rate of the acid number (rav) has been calculated by the experimental curves AN = f(t), which may be regarded, as the average velocity of the reaction.
The statistical analysis of the reaction rate dependence from the process parameters has been used for the mathematical data processing — the paraffin chlorination and the catalyst concentration degrees. It, moreover, has been found, that all the experimental data (e.g. in the selected range of the catalyst concentrations and the paraffin chlorination degree) are being described by the linear mathematical dependence and its relationship for the following values of the equation coefficients:
![]()
where AN – the acid number;
К1 – the average initial acid number of the reaction mass, which is equal to 3,941;
К2 – the constant, which is equal to 2,092;
rav – the average accumulation rate of the acid number (AN);
CKt – the catalyst concentration (e.g. % weight).
The mathematic expression view in the implicit form of the rate rav dependence of the catalyst concentration and the paraffin chlorination degree have already been established with the regression analysis using, which has been shown, that the rate reaction is described by the linear dependence and its relationship with the satisfactory precision (e.g. the average standard square deviation is made up ~ 1%) (Figure 2).
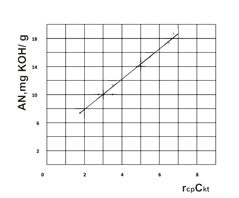
The Figure 2. The acid number (AN) dependence of the control parameters.
![]()
At the following regression coefficients values:
a – the regression coefficient, which is equal to 0,01131;
b – the regression coefficient, which is equal to 1,07594;
с – the regression coefficient, which is equal to 0,03475;
nCl – the hydrocarbon chlorination degree (e.g. % weight);
CKt – the catalyst concentration (% weight).
The statistical analysis of the obtained results has been carried out for the AN dependence establishment of the control parameters in the process of the chlorinated paraffins oxidation by the air oxygen, by means of the Statistical Package using for the applied program application of the DataFit 8.0 computer (Figure 3).
So, the already found dependence of the initial parameters acid number (AN) can be presented in the following form:
![]() ,
,
which is the control one, and it is allowed to be calculated the acid number (AN), having set the catalyst initial concentration, and the paraffin chlorination degree. Since the acid number (AN) is practically depended on the catalyst concentration and the accumulation rate of the acid number (AN) over the time (which, in its turn, is practically depended on the catalyst concentration), then the resulting value itself of the acid number (AN) is very dependent on the catalyst concentration to the second power.
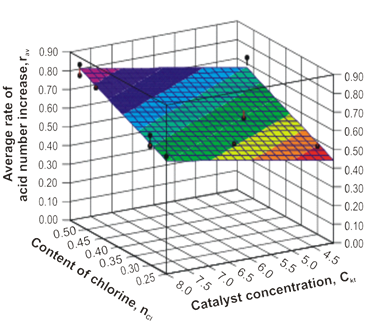
The Figure 3. The average rate dependence of the acid number increase of the Ckt catalyst concentration and the nCl hydrocarbon chlorination degree.
The adequacy of the obtained mathematical dependence and its relationship to the experiment has already been tested by the additional independent experiment on the CHP-40 brand chlorinated paraffin oxidation. But the special experiments on this chlorinated paraffin oxidation, at the data preparation for the mathematical treatment, have not been carried out yet. For the experiment, the Ckt value, different from those, used in the data processing, has been selected to be adequacy tested. Previously, by the suggested mathematical dependence and its relationship, it has been calculated the AN (e.g. the paraffin chlorination degree nCl= 40% (e.g. weight), the catalyst concentration Ckt =5% (e.g. weight)). The acid number (AN) value has been made up AN = 10,4 mg KOH/g, having calculated, according to the equation. So, the CHP-40 chloroparaffin has already been oxidized in the special additional experiment, under the following conditions: the temperature of 107-108ºС, the catalyst amount of 5% (e.g. weight), the air flow rate of 6 l/( min·kg) for 20 hours. Then, the oxidate has been obtained by us with the average acid number of 10,6 mg KOH/g. The adequacy dispersion of the supposed mathematical model has been made up 3,6% for these indicated conditions.
Thus, the adequacy of the obtained mathematical dependence and its relationship to the experiment in the selected range of the parameters has already been confirmed.
On the example of the CHP-30 industrial chlorinated paraffin oxidation, it has already been found, that the stearate – anion presence in the studied catalytic system is practically provided to be reached the increased acid concentration in the oxidation products, as compared with the catalyst, having contained only the acetate – anion.
2. http://cameochemicals.noaa.gov/chris/DOP.pdf;
3 Pat. .№ 2227795 RF, MPC C 07 С 53/19. “The Production Method of Higher Fatty Chlorinated Acids”./ B.E. No, Yu.L. Zotov, A.V. Gora. «The Volgograd State Technical University»: Appl. 04.11.02.; Publ. 27.04.04;
4. Pat. № 2323234 RF MPC C 08 L 27/00, С 08 К 13/02. “The Polymer Composition for Products”. / Yu.L. Zotov, K.F. Krasilnikov, Yu.V. Popov, A.V. Gora, N.A. Butakova, N.N. Tairova: «The Volgograd State Technical University»: Appl. 09.01.07; Publ. 27.04.08;
1. Pat. 2295549 RF, MPC C 08 L 27/06, С 08 К 13/02. “The Polymer Composition for Products”. / Yu.L. Zotov, K.F. Krasilnikov, Yu.V. Popov, E.V. Erina: «The Volgograd State Technical University»: Appl. 10.01.06; Publ. 20.03.07;
6. Emanuel, N.E. ,“ The Chain Reactions of Hydrocarbons Oxidation in Liquid Phase”. / N.E. Emanuel. – М.: «Science», 1965 – p. p. 362;
7. Steven Blaine. ,”Reaction Pathways in Lubricant Degradation. 1. Analytical Characterization of n-Hexadecane Autoxidation Products”. /Steven Blaine, Phillip E. Savage // «Ind. Eng. Chem. Res.» – 1991. –Vol. 30.–P. P.792 – 798;
8. Steven Blaine. ,”Reaction Pathways in Lubricant Degradation. 2. n-Hexadecane Autoxidation”./ Steven Blaine, Phillip E. Savage // «Ind. Eng. Chem. Res.» –1991. –Vol. 30. – P. P. 2185 – 2191;
9. Dagaut, P.,”Chemical Kinetic Study of the Oxidation of Isocetane (2,2,4,4,6,8,8,-Heptamethylnonane) in a Jet-Stirred Reactor: Experimental and Modeling”. / P. Dagaut, K. Hadj-Ali // «Energy & Fuels.» – 2009. – V. 23. – P. P. 2389 – 2395;
10. Ranzi, E. ,”Wide-Range Kinetic Modeling Study of the Pyrolysis, Partial Oxidation and Combustion of Heavy n-Alkans”. / E. Ranzi [et. al.] // «Ind. Eng. Chem. Res.» – 2005. – V. 44. – P.P. 5170 – 5183;
11. Biet, J. ,”Experimental and Modeling Study of the Low-Temperature Oxidation of Large Alkanes”. / M. H. Hakka [et al.] // «Energy & Fuels.» – 2008. – V. 22. – P.P. 2258 – 2269;
12. Pfaendther, J. ,”Mechanistic Modeling of Lubricant Degradation. 1. Structure-Reactivity Relationships for Free-Radical Oxidation”. / J.Pfaendther, L. J Broadbelt. // «Ind. Eng. Chem. Res.» – 2008. – V. 47. – P.P. 2886 – 2896;
13. Pfaendther, J. ,”Mechanistic Modeling of Lubricant Degradation. 2. The Autoxidation of Decane and Octane”. / J.Pfaendther, L. J Broadbelt// «Ind. Eng. Chem. Res.» – 2008. – V. 47. – P. P. 2897 – 2904;
14. Garcia-Ochoa, F. ,”Modeling of the Thermal n-Octane Oxidation in the Liquid Phase”. / F. Garcia-Ochoa, A. Romero, J. Querol // «Ind. Eng. Chem. Res.» –1989. – Vol. 28. – P.P. 43 – 48;
15. Boss, B.D. ,”Oxidation of Hydrocarbons in the Liquid Phase: n-Dodecane in a Borosilicate Glass Chamber at 2000C”. / B.D. Boss.; R.N. Hazlett// «Can. J. Chem.» – 1969. – 47. –P.P. 4175 – 4182;
16. Kharitonov, V.V. ,”The Reaction Medium Self – Structuring Influence on the Mechanism of n-Heptadecane Deep Oxidation”./ V.V. Kharitonov // «The Petrochemicals». – 2003 – Vol. 43. – №2. – P.P. 97 – 104;
17. Bakunin, V.N. ,”The Structure Change of Hydrocarbon Medium in the Process of Liquid-Phase Oxidation”. / V.N. Bakunin [et. al.] // «The Petrochemicals». – 2001 – Vol. 41. – №1. – P.P. 41 – 47;
18. Oganesova, A.Yu. ,”The Conditions Effect of Liquid-Phase High Temperature Hexadecane on the Process Mechanism”. / A.Yu. Oganesova [et. al.] // «The Petrochemicals». – 2004. – Vol. 44. – №2. – P.P. 119 – 126;
19. Oganesova, A.Yu. ,”The Structure Effect of Higher Paraffinic Hydrocarbons and Their Derivatives on the High-Temperature Liquid-Phase Oxidation Mechanism”. / A.Yu. Oganesova [et. al.] // «The Petrochemicals». – 2009 – Vol. 49. – № 4. – P.P. 329 – 334;
20. Butakova, N. А.,”Complex Additives for Poly(vinyl chloride) Processing Based on Chloroparaffins”. / N. А. Butakova, Y. L. Zotov, К. V. Sidorov // Modern Problems of Polymer Science Progr.: Abstract Book of the 5-th Saint-Petersburg Young Scientists Conf. (St.-Petersburg, October, 19–22, 2009) / «The Institute of Macromolecular Compounds RAS [et. al.].» – St.-Petersburg, 2009 – P. 102;
21. “The Study of Catalysts Influence, Having Contained Metals Ions of Variable Valence on the Process of the Liquid Chlorinated Paraffins Oxidation”. / N.A. Butakova [et al.] // «The High Chemical Technologies-2006: The Proc. Reports. The XI-th International Scientific and Technical Conference, October, 16-20, 2006.» / The Samara State Technical University [et. al.] – Samara, 2006, – Vol.I. – P.P. 99 – 100;
22. Zotov, Y.L. ,”Plasticizing Compositions for Polyvinylchloride Processing on the Basis of Chloroparaffin CP-30 Oxidation Products”. / Y.L. Zotov, N.A. Butakova, E.K. Zakharova // «The XIX-th Mendeleev Congress on General and Applied Chemistry: Abstract Book in 4 volumes». – Volgograd, 2011. – Vol.2. – P. 644;
23. Akkihebbal Krishnamurthy Suresh. ,”Engineering Aspects of Industrial Liquid-Phase Air Oxidation of Hydrocarbons / Akkihebbal Krishnamurthy Suresh, Man Mohan Sharma, Tamarapu Sridhar. // «Ind. Eng. Chem. Res.» –2000. – Vol. 39, P.P. 3958 – 3997.
Zotov Yu.L., Gora A.V., Butakova N.A., Volchkov V.M., Popov Yu.V. THE INDUSTRIAL CHLORINATED PARAFFINS OXIDATION BY AIR IN THE
PRESENCE OF MANGANESE SALTS AND THE PROCESS CONTROL PARAMETERS
. International Journal Of Applied And Fundamental Research. – 2013. – № 1 –
URL: www.science-sd.com/452-24447 (11.02.2026).









 PDF
PDF