About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Medical sciences
Introduction
The medical films containing various drugs were widely applied in dental therapy. The prolonged effect in such films is reached by the immobilization of drugs on various polymeric carriers [1]. The main advantage of medicinal films is possibility of the programmed delivery of drug by regulation of the nature of polymeric matrix. Such films when placing on mucous oral cavities are capable to release slowly anesthetizing drug, providing long local anesthesia. Using various methods it is possible to change the physical and chemical properties of polymer matrix and respectively the release of drug [2].
The purpose of present work is development of novel dosage forms with the controlled action by immobilization of local anesthetics on polymeric films. Local anesthetics lidocaine and novocaine are used as drugs. The drug release characteristics of such systems are discussed.
Material and methods
The local anesthetics lidocaine and novocaine were used pharmaceutical grade. Polyvinyl alcohol (PVA) with ММ 70 000 was purchased from Sigma Chemicals, St. louis, USA.
Polymeric films are received from the corresponding solutions of polymer and drug by water evaporation. The amount of PVA is filled with distilled water and maintained on magnetic mixer at temperature 80-90°C before full dissolution. The calculated amount of drug added to cooling at room temperature homogeneous solution of PVA. After stirring the received solution poured out in glass established horizontally and dried in at room temperature up to constant weight. The received medicinal form had an appearance of thin elastic transparent film from which by means stamp cut out squares 0,2-0,5 mm thick. Calculation of dose of drug was carried out from criterion of the minimum dose. The release behaviour of drug from polymeric samples was examined by means of immersing the disc-shaped samples of 0,3-0,5 mm thickness and 10,0 mm diameter in a Ringer-Lock solution at 37°C. The amount of drug released was determined by UV-spectrometry by measuring the absorption maximum. UV spectra were recorded on a Jasco UV/VIS-7850 (Japan) spectrophotometer.
Results and Discussion
Polyvinyl alcohol is widely used as polymeric carriers in medicinal films. This polymer already found application in medicine as emulsifier, thickener and stabilizer of suspensions, filming agent for capsules and tablets, bases for ointments [3]. We developed medical films on the basis of PVA containing various doses of local anesthetics lidocaine and novocaine. Release of drug in conditions in vitro is investigated by means of the UV-spectroscopy method. It is shown that the drug which has been evenly dispersed in polymer is released on model solution on the diffusion mechanism with rate reduction. Process of diffusion is described by Fick's law and occurs according to kinetics of the first order. Data on drug release from polymeric films are presented in Figure.
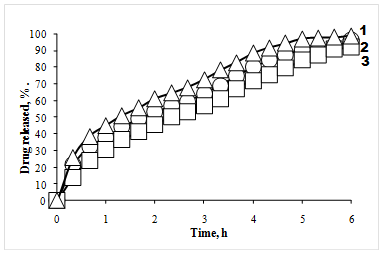
Figure - Release of lidocaine from PVA-films at various drug loading: 25 mg/1 g of PVA (1), 50 mg /1g of PVA (2), 100 mg /1 g of PVA (3)
It is established that release process of lidocaine from films in environment consists of three main stages: 1) water sorption by polymeric film and its swelling; 2) diffusion of drug in film on phases polymeric system - environment; 3) diffusion of drug in solvent volume. Drugs almost completely diffused from PVA-films within 6-8 hours, without undergoing any changes. With increase in thickness of film the process of diffusion of drug is slowed down. The increase in loading of drug leads to delay of lidocaine diffusion rate from the film.
All the release data show the typical pattern for a matrix controlled mechanism. The cumulative amount of drug released from films was linearly related to the square root of the time and the release rate decreased with time. Duration of drug release from monolithic therapeutic systems considerably depends on the swelling of polymeric matrix. Dependence swelling degree of PVA-films from thickness of samples shown that the most optimum properties films 0,4-0,6 mm thick possessed. Such materials swelled for 55-60% within initial 1,5-2,0 hours with the subsequent achievement of the maximum value of 80% in 6 hours, thicker films very slowly swelled for 45-50% within 1,5-2 hours that didn't conform to medical requirements. Release of drug is limited by the rate of swelling and thickness of polymeric matrix.
Clinical observations on patients with periodontal disease inflammatory and inflammatory-destructive nature showed significant advantages of using polymer film forms of lidocaine. Clinical efficacy was confirmed in statistically significant reduction of terms of treatment of patients with generalized periodontal disease, improvements of the test of Kulagina, gingival index of Loe, hygienic condition of the mouth, a higher percentage of remission of the disease in the early and late periods.
Conclusion
Polymer film dosage forms of local anesthetics have been developed. Drug release from polymeric forms is investigated. Drug containing polymeric films shows a high initial release rate and the matrix-controlled release for more 6-8 h. The release data depends on drug loading and polymer structure. The possibility of application of polyvinyl alcohol for prolongation of anesthetics is show.
2. Ishuda V., Nambu N., Nagai T. Mucosal dosage form of lidocaine for toothache using hydroxypropyl cellulose and carbopol // Chem. And Pharm. Bull. – 1982. – № 3. –P.980-984.
3. Aleyamma A.J., Sharma Ch.P. Polyvinyl Alcohol as a biomaterial // Blood compatible materials and devices. Ed.by Sharma Ch.P.& Szycher M.Technomic Publ. 1991. – P.123-130.
Batyrbekov E.O., Dalabaeva N.M. DEVELOPMENT OF POLYMER FILM DOSAGE FORMS OF LOCAL ANESTHETICS. International Journal Of Applied And Fundamental Research. – 2016. – № 2 –
URL: www.science-sd.com/464-25154 (04.03.2026).









 PDF
PDF