About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Medical sciences
Particular attention in recent years attracts an increased incidence of depressive states and associated with them anxious, phobic, obsessive and somatoform disorders, which are one of the major mental health problems of children and adolescents in the world, including Ukraine [3, 4].
It is a matter of common knowledge that in formation of depressions a significant part belongs to sociodemographic, psychological and family factors that correlate with age, gender, ethnicity, socioeconomic status, family dysfunction type, summated life stress, the presence of psychopathology in the parents, a low intellectual level, the presence of somatic diseases, and a low self-appraisal level. Special place in the formation of these conditions is assigned to genetic factors. Thus, the metabotropic glutamate receptor 7, which is involved in the formation of depression, is localized in the 3p25-26 chromosome [12]. It is assumed that certain genes, located in other chromosomes (2p11-q14, 4p16, 12q23-24, 13q21-33, 15q25.5-26.2, 16p13, 21q22, and Xq24-26) are also implicated in the depression occurrence which testifies to the necessity of profound cytogenetic study [5-11].
The aim of the study: to investigate spontaneous and induced mutagenesis in the peripheral blood lymphocytes of children with depression.
Materials and methods
Cytogenetic analysis has been carried out in 21 patients with depression before and after administration of mitomycin C, and in 24 healthy coevals, aged 6 to 17, who were under clinical research in the State Institution "ICAHC NAMS of Ukraine."
Culture of peripheral blood lymphocytes was performed by a standard procedure [2]. Materials for the cytogenetic analysis were chromosomal preparations obtained from the culture of peripheral blood lymphocytes (PBL). To assess the impact of the mutagen on the chromosomal apparatus stability in probands mitomycin C in a final concentration of 3 mcg/ml was added to the mixed culture on the 67th hour of incubation. Three hours before fixation colchicine in a final concentration of 7.5 mcg/ml was added into the cellular culture [1]. Staining of chromosomal preparations: homogeneous and differential (GTG- method).
50 to 100 metaphases from each child were analyzed without testing impact but with an additional mutagenic pretreatment of the cultures in vitro. 2100 metaphase plates, obtained from patients with depression, were analyzed before their treatment with mitomycin C, and 1966 plates – after their exposure to the mutagen in vitro; 921 plates, obtained from healthy patients, were analyzed before exposure to mitomycin C, and 1947 plates – after their pretreatment, respectively. All the disorders of chromatid, chromosomal and genomic types were taken into account. Metaphase plates were examined using a Leica CME binocular microscope (Austria), 10X18 eyepiece, 100x lens, and 1.25 x binocular head.
Statistical processing of the obtained results was performed on a PC using the Excel and SPSS Statistics 17,0 software packages. Student's t test was used to identify the significance of differences between compared findings.
Results and discussion
According to the results of cytogenetic analysis, karyotype in all patients with depression corresponded to the normal female – 46,XX or male – 46,XY. It is well-known that at the cytogenetic level an important role in the destabilization of the human genome plays latent chromosomal instability in normal karyotype, which manifests itself as chromosomal hypersensitivity of peripheral blood lymphocytes to the action of other mutagens – in vivo and in vitro. The total number of chromosome aberrations in patients with depression before the impact of mutagen-provocateur mitomycin C came to 13.1 %, whereas in healthy probands it was 1.81 %, p<0,001. After exposure of PBL to the mutagen- provocateur’s impact the incidence of chromosomal abnormalities in patients increased by 1.6 times (21.5 %, p <0,001) and in healthy children – by 8.6 times (15.6 %, p <0,001). Comparison of spontaneous and induced mutagenesis levels in our patients allowed the authors to establish an increase in chromosomal aberrations number (chromatid and chromosomal types) after exposure to mitomycin C (18.2 % vs. 10.1 %, p<0.001) (table).
Single fragments prevailed among chromatid-type aberrations, and among chromosome-type they were elongation and breaks on the centromere and chromatid -isochromatid exchanges, while after mitomycin C mutagenic impact on PBL total frequency of the genomic-type disorders did not change. Only a twofold increase in the number of metaphase plates with premature divergence of centromere has been registered in the study (p<0.05).
Analysis of individual frequencies as regards PBL hypersensitivity to clastogenic action of mutagen-provocateur in sick children revealed that the level of chromosomal aberrations in probands with depression before exposure to mitomycin C in vitro ranged from 5.0 % to 26.0 %, and after mutagenic load – from 13.0 % to 36.0 % (Fig.).
In healthy probands parameters of the kind increased from 0.0 % to 4.5 % before the in vitro mutagen impact, and after exposure to mitomycin C – from 3.0 % to 33.7 %, respectively, i.e. a significant increase in the individual level of the induced mutagenesis had been registered in both compared groups.
Table
The incidence of chromosome aberrations in patients with depression before and after mutagenic load with mitomycin C, % ± m
|
Aberration types |
Before load, n = 21 |
After load, n = 21 |
Р |
|
Chromatid type: single fragments short arm deletion+ long arm deletion |
6.33 ± 0.33 4.90 ± 0.47 1.43 ± 0.18 |
8.70 ± 0.64 6.56 ± 0.56 2.14 ± 0.44 |
<0.001 <0.05
>0.05 |
|
Chromosome-type: paired fragments centromere elongation centromere breaks chromatid–isochromatid exchanges |
3.76 ± 0.42 2.0 ± 0.31 1,0 ± 0.22 0.52 ± 0.16 0.0 ± 0.0 |
9.46 ± 0.66 1.98 ± 0.32 3.1 ± 0.39 1.83 ± 0.30 0.76 ± 0.19 |
<0.001 >0.05 <0.001 <0.001 <0.001 |
|
Genome-type: polyploid cells premature centromere divergence |
2.95 ± 0.37 2.19 ± 0.32 0.76 ± 0.19 |
3.36 ± 0.41 1.83 ± 0.30 1.53 ± 0.28 |
>0.05 >0.05
<0.05 |
|
Total frequency of disorders |
13.1 ± 0.74 |
21.5 ± 0.93 |
<0.001 |
|
Notice. n – number of patients; P – level of significance. |
|||
However, the study of the individual overspontaneous mutagenesis has shown a significant increase in this parameter in healthy individuals, indicating a decreased adaptive response in probands with depression.
Thus, it has been found a significant increase in spontaneous and induced mutagenesis in PBL of patients, as compared with healthy individuals, which testifies to the expressed chromosomal instability of the genome in sick children.
As a result of cytogenetic studies there were identified spontaneous and induced mutagenesis levels in children with depressive states. The total number of chromosome aberrations before the impact of mutagen-provocateur mitomycin C in patients with depression came to 13.1 %, whereas in healthy probands it was 1.81 %, p <0,001. After exposure of PBL to the mutagen-provocateur impact the frequency of chromosome abnormalities in patients increased by 1.6 times (21.5 %, p<0,001) and in healthy children by 8.6 times (15.6 %, p<0,001), indicating a decreased adaptive response in children with depression. Single fragments prevailed among chromatid-type aberrations in patients; among chromosome-type they were elongation and breaks on the centromere and chromatid-isochromatid exchanges; among genome type – premature centromere divergence, as compared with the frequency of those disorders in healthy children.
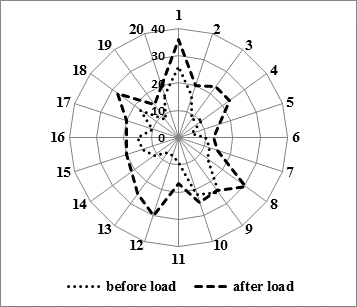
Fig. Analysis of an individual peripheral blood lymphocytes hypersensitivity in patients with depression before and after exposure to mitomycin C,%
2. Zerova-Lyubimova T.E., Gorodenko N.G. Cytogenetic methodological investigation of human chromosomes: Method. Instructions. – P. Shupyk KMAPE, K., 2003. – 23 p.
3. Podkorytov V. S. Depressions. Current therapy: a guide for physicians. – Kh.: Tornado, 2003. – 352 p.
4. Shchastny E.D. Anxiety and depressive disorders in students in the system of primary vocational education [electronic resource] // Medical psychology in Russia: electron. scient. journal. – 2012. – N.3 (14). – Mode of access to the journal. URL: http://medpsy.ru.
5. Craddok N., Jones I.J. Genetics of bipolar disorder // Med. Genet. – 1999. – Vol. 36. – P. 585-594.
6. Hamilton S.P. A new lead from genetic studies in depressed siblings: assessing studies of chromosome 3 // Am. J. Psychiatry. – 2011. – Vol.168 (8 ). – P. 783-789 .
7. Holmans P. [et al.]. Genomewide Significant Linkage to Recurrent, Early-Onset Major Depressive Disorder 15q // Am. J. Genet. – 2004. – Vol.74. – P. 1154-1167 .
8. Goes F.S., Zandi P.P., Miao K. [et al.]. Mood-incongruent Psychrotic Features in Bipolar Disorder: Familian Aggregation and Suggestive Linkage to 2p11-q14 and 13g21- 33 // Am. J. Psychiatry. – Vol.164. – P. 2007. – P.236- 237.
9. Kaplan M.I., Limoli C.L., Morgan W.F. Perpetuating radiation-induced chromosomal instability // Radiat. Oncol. Invest. – 1997. – Vol.5. – P.124- 128.
10. Levinson D.F., Evgrafov O.V., Knowles J.A. [et al.]. Genetics of recurrent early-onset major depression (GenRED): Significant linkage on chromosome 15q25-q26 after fine mapping with single nucleotide polymorphism markers // Am. J. Psychiatry. – 2007. – Vol. 164. – P. 259-264.
11. Middeldorp S.C.M., Sullivan P.F., Wray N.R. [et al.]. Suggestive Linkage on Chromosome 2, 8, and 17 for Lifetime Major Depression // Am. J. Med. Genet. B. Neuropsychiatr. Genet. – 2009. – 150B (3). – P. 352-358.
12. Pergadia M.L., Glowinski A.L., Wray N.R. [et al.]. A 3p26-3p25 genetic linkage finding for DSM-IV major depression in heavy smoking families // Am. J. Psychiatry. – 2011. – Vol. 168 (8). – P. 848-52.
Bagatskaya N.V., Sweedan Enass Gh. SPONTANEOUS AND INDUCED MUTAGENESIS PECULIARITIES IN PERIPHERAL BLOOD LYMPHOCYTES OF PATIENTS WITH DEPRESSIVE DISORDERS. International Journal Of Applied And Fundamental Research. – 2014. – № 1 –
URL: www.science-sd.com/456-24475 (18.02.2026).









 PDF
PDF