About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Materials of the conference "EDUCATION AND SCIENCE WITHOUT BORDERS"
Abstract—The thermodynamics of tapping, dephosphorization, and out-of-furnace treatment of steel in a ladle and the related phosphorus balance are considered, and the main technological and thermodynamic parameters for decreasing the metal dephosphorization are found.
SPELL: 1. slagless, 2. slagging
The contents of phosphorus and sulfur in a metal during deoxidation, tapping, inert gas blowing, and casting can increase gradually due to their introduction with deoxidizers and reduction from a slag. This circumstance should be taken into account to maintain dephosphorization conditions for a metal.
As a result of reactions between a metal, slag, and deoxidizers, phosphorus equilibrium shifts toward an increase in the phosphorus content in a metal despite a significant decrease in the temperature. In this case, dephosphorization depends substantially on the ratio of the metal to slag weight. The role of these factors can be deduced from the following phosphorus balance calculation: (phosphorus content in ladle slag) + (phosphorus content in ladle metal) = (phosphorus
content in tapped metal) + (phosphorus content in slag trapped by metal in ladle) + (phosphorus from deoxidizers).
The balance equation is
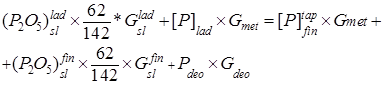 , (1)
, (1)
where ![]() is the phosphorus content in the ladle (%);
is the phosphorus content in the ladle (%);![]() is the phosphorus in the metal before tapping from the steelmaking unit into the ladle (%);
is the phosphorus in the metal before tapping from the steelmaking unit into the ladle (%);![]() is the phosphorus content in deoxidizers;
is the phosphorus content in deoxidizers; ![]() and
and ![]() are the
are the ![]() contents in the ladle and final slags (%), respectively;
contents in the ladle and final slags (%), respectively; ![]() and
and ![]() are the quantities of the ladle and final slags trapped by the metal (t); and
are the quantities of the ladle and final slags trapped by the metal (t); and ![]() and
and ![]() are the quantities of the metal and deoxidizers (t).
are the quantities of the metal and deoxidizers (t).
We divide all terms in Eq. (1) by ![]() and
and ![]() (62/142 = 0.4366,
(62/142 = 0.4366, ![]() = 0); introduce transformations
= 0); introduce transformations ![]() /
/![]() =
=![]() ,
, ![]() /
/![]() =
=![]() ,
, ![]() /
/![]() =
= ![]() , and
, and ![]() /
/![]() =
=![]() ; solve the equation for phosphorus in the ladle metal; and obtain the expression
; solve the equation for phosphorus in the ladle metal; and obtain the expression
![]() , (2)
, (2)
where ![]() /
/![]() =
=![]() is the coefficient of phosphorus distribution between the ladle slag and the ladle metal;
is the coefficient of phosphorus distribution between the ladle slag and the ladle metal; ![]() /
/![]() =
=![]() is the coefficient of phosphorus distribution between the final converter slag, the slag entering into the ladle, and the metal;
is the coefficient of phosphorus distribution between the final converter slag, the slag entering into the ladle, and the metal; ![]() /
/![]() =
= ![]() is the ratio of the ladle slag to the metal; and
is the ratio of the ladle slag to the metal; and ![]() /
/![]() =
=![]() is the ratio of the converter slag to the metal.
is the ratio of the converter slag to the metal.
Expression (2) can be rewritten as
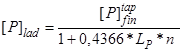 , (3)
, (3)
if we consider an ideal case, when tapping of metal from the steelmaking unit into the ladle occurs with complete slag cutoff and the penetration of the final converter slag is excluded (![]() ).
).
It is seen that the phosphorus content in the ladle metal decreases with increasing coefficient of phosphorus distribution ![]() between the induced ladle slag and the metal after heat deoxidation and with increasing slag ratio n. However, the conditions of slagless metal tapping cannot be reached under real industrial conditions.
between the induced ladle slag and the metal after heat deoxidation and with increasing slag ratio n. However, the conditions of slagless metal tapping cannot be reached under real industrial conditions.
If we take into account that the ladle slag is averaged when the final slag penetrates into the ladle, we can conventionally assume that ![]() =
= ![]() =
= ![]() . Then, Eq. (3) can be rewritten as
. Then, Eq. (3) can be rewritten as
![]() , (4)
, (4)
where ![]() –
–![]() characterizes the degree of ladle slag dilution and
characterizes the degree of ladle slag dilution and
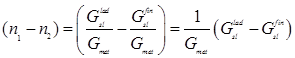 , (5)
, (5)
The figure shows the effect of slag dilution ![]() at two values of
at two values of ![]() (
(![]() = 0.5 × 104 for current heats and
= 0.5 × 104 for current heats and![]() =1.5 × 104 for the case when a mixture of lime and fluorite is added to the ladle). As is seen from the figure, metal dephosphorization decreases with increasing
=1.5 × 104 for the case when a mixture of lime and fluorite is added to the ladle). As is seen from the figure, metal dephosphorization decreases with increasing![]() even at the same degree of ladle slag dilution. Moreover, even at the same degree of ladle slag dilution, a decrease in the quantity of slag entering into the ladle
even at the same degree of ladle slag dilution. Moreover, even at the same degree of ladle slag dilution, a decrease in the quantity of slag entering into the ladle ![]() favors metal dephosphorization due to the refining capacity of the ladle slag.
favors metal dephosphorization due to the refining capacity of the ladle slag.
Thus, the theoretical analysis of the metal dephosphorization in a ladle demonstrates that phosphorus is reduced due to a decrease in the ladle slag basicity and the presence of ferrous oxide in this slag. An analysis of the balance of the slag that enters into the ladle in metal tapping from the converter shows that the main quantity of slag is trapped into the ladle by the funnel that forms in tapping, and the minimum quantity of slag is 1–2 t even for the most effective slag cutoff methods.
One of the most efficient methods for decreasing dephosphorization is the dilution of the ladle slag. However, this method is especially effective if acid materials pas to a thick basic slag layer, where they are strongly diluted.
Equation (4) shows that the larger the quantity of the final slag in the ladle in tapping, the higher the degree of reduction of phosphorus from the ladle slag to the metal and the higher the phosphorus concentration. The ![]() content in the ladle slag depends on the quantity of the final slag that passes to the ladle during tapping and on the
content in the ladle slag depends on the quantity of the final slag that passes to the ladle during tapping and on the ![]() content in this slag. It was found that the higher the coefficient
content in this slag. It was found that the higher the coefficient ![]() /
/![]() =
=![]() by tapping and the beginning of heat deoxidation, the higher the degree of phosphorus reduction (dephosphorization) from the slag to the metal during deoxi ation. As a rule, an increase in the
by tapping and the beginning of heat deoxidation, the higher the degree of phosphorus reduction (dephosphorization) from the slag to the metal during deoxi ation. As a rule, an increase in the ![]() content in the final slag by more than 5%
content in the final slag by more than 5%
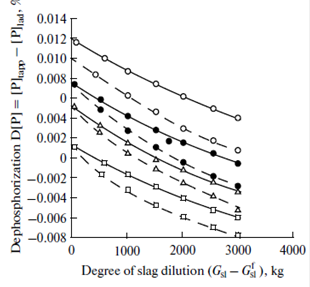
Effect of the degree of slag dilution on the metal dephosphorization in a ladle:
(![]() )
)![]() = 0.5 × 104, (
= 0.5 × 104, (![]() )
) ![]() =1.5 × 104, () (
=1.5 × 104, () (![]() ) = 1000 kg,
) = 1000 kg,
![]() (
(![]() ) = 2000 kg,
) = 2000 kg, ![]() (
(![]() ) = 3000 kg, and
) = 3000 kg, and ![]() (
(![]() ) = 4000 kg.
) = 4000 kg.
leads to an increase in the phosphorus concentration by 0.004–0.014%. A decrease in the ![]() content in the final slag is achieved due to the maximally complete removal (slagging) of
content in the final slag is achieved due to the maximally complete removal (slagging) of ![]() - rich intermediate slag in the first stage of heat.
- rich intermediate slag in the first stage of heat.
If the reaction of phosphorus oxidation is written as
![]() , (6)
, (6)
we have
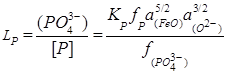 , (7)
, (7)
An increase in ![]() in the steelteeming ladle is achieved by an increase in the ferrous oxide activity in slag
in the steelteeming ladle is achieved by an increase in the ferrous oxide activity in slag ![]() and the oxygen activity in slag
and the oxygen activity in slag ![]() and by a decrease in the activity coefficient of
and by a decrease in the activity coefficient of ![]() in slag. Taking into account that the metal temperature in the ladle changes over a narrow range (1550–1570°C), we assume that
in slag. Taking into account that the metal temperature in the ladle changes over a narrow range (1550–1570°C), we assume that ![]() is constant. To exclude the passage of oxygen from the slag to the metal and secondary oxidation reactions in the ladle during inert (neutral) gas blowing of the metal, it is necessary to maintain the ferrous oxide activity in slag
is constant. To exclude the passage of oxygen from the slag to the metal and secondary oxidation reactions in the ladle during inert (neutral) gas blowing of the metal, it is necessary to maintain the ferrous oxide activity in slag ![]() or the oxygen activity in slag
or the oxygen activity in slag ![]() at the lowest level. The only method for increasing
at the lowest level. The only method for increasing ![]() is a decrease in the
is a decrease in the ![]() activity coefficient in slag, which is possible if it is fixed by strong calcium oxide phosphates and is achieved by an increase in the calcium oxide activity in the ladle slag.
activity coefficient in slag, which is possible if it is fixed by strong calcium oxide phosphates and is achieved by an increase in the calcium oxide activity in the ladle slag.
In tapping the metal into the ladle during oxidation and holding the metal in the ladle, the slag basicity and the calcium oxide activity decrease because of the negative effect of silicon oxide that enters into the slag due to the erosion of the ladle lining and deoxidizers. The reduction of phosphorus during an increase in the (SiO2) content is explained by the fact that stronger anion ![]() occupies certain positions near cations (Ca2+, Mg2+, Mn2+, Fe2+, etc.), which were earlier occupied by weaker anion
occupies certain positions near cations (Ca2+, Mg2+, Mn2+, Fe2+, etc.), which were earlier occupied by weaker anion![]() As a result of this replacement, the bonds of anion
As a result of this replacement, the bonds of anion ![]() with the slag weaken and, hence, part of phosphorus passes from the slag to the metal.
with the slag weaken and, hence, part of phosphorus passes from the slag to the metal.
A decrease in the degree of dephosphorization and the phosphorus concentration in the ladle metal can be achieved by increasing the degree of dilution of the ladle slag, which decreases the SiO2 and ![]() contents. This is reached by the addition of lime to the ladle in tapping. However, this technique cannot always gives expected results, since it often leads to the thickening of the ladle slag. However, the thickening of the slag in the ladle does not hinder the passage of phosphorus from the slag to the metal. A thin slag layer exists at the slag–metal interface, and it determines the phosphorus distribution between the metal and slag. A strongly acid contact slag layer, which mainly consists of the products of deoxidation and erosion of the acid ladle lining, forms under the thickened and solidified slag layer in casting. The detrimental effect of this slag can be eliminated only in the case if acid materials pass to a thick layer of a liquid basic slag and dilute it strongly. This serves as the basis of a successful application of dephosphorization mixtures of lime and fluorite or another diluting component, e.g., a material containing Al2O3 or B2O3 or the materials forming these oxides during interaction with metal or slag oxygen. The application of diluting materials mainly increases the calcium oxide activity due to the dissolution and assimilation of added lime by slag, increases the ladle slag basicity, and increases the coefficient of phosphorus distribution between the ladle slag and the metal.
contents. This is reached by the addition of lime to the ladle in tapping. However, this technique cannot always gives expected results, since it often leads to the thickening of the ladle slag. However, the thickening of the slag in the ladle does not hinder the passage of phosphorus from the slag to the metal. A thin slag layer exists at the slag–metal interface, and it determines the phosphorus distribution between the metal and slag. A strongly acid contact slag layer, which mainly consists of the products of deoxidation and erosion of the acid ladle lining, forms under the thickened and solidified slag layer in casting. The detrimental effect of this slag can be eliminated only in the case if acid materials pass to a thick layer of a liquid basic slag and dilute it strongly. This serves as the basis of a successful application of dephosphorization mixtures of lime and fluorite or another diluting component, e.g., a material containing Al2O3 or B2O3 or the materials forming these oxides during interaction with metal or slag oxygen. The application of diluting materials mainly increases the calcium oxide activity due to the dissolution and assimilation of added lime by slag, increases the ladle slag basicity, and increases the coefficient of phosphorus distribution between the ladle slag and the metal.
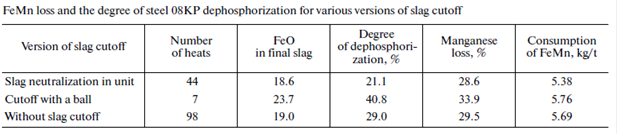
The tests of various methods of slag cutoff (cone gates and floating stoppers) showed their low efficiency and did not exclude slag penetration into the ladle in metal tapping. The best version for the most complete limitation of slag penetration into the ladle is slag neutralization before heat tapping by thickening at the last stage of heat. The neutralization of the final slag in the unit makes it possible to exclude slag penetration into the ladle or to achieve the slag reactivity at which the reduction of phosphorus is limited (table).
The data on dephosphorization and the ferroalloy loss are given in the table. Experimental heats performed with slag neutralization in a converter before tapping demonstrate a decrease in the slag mass in the steel-teeming ladle and a decrease in the ferrous oxide mass in the final slag by 2.7–4.9%. Our investigations show that a decrease in the reduction of phosphorus is achieved by the maintenance of a high basicity and refining capacity of the ladle slag and the introduction of various dephosphorization slagforming mixtures in the ladle during tapping.
Ibraev I. K., O. T. Ibraeva Problems of Dephosphorization during Steelmaking. International Journal Of Applied And Fundamental Research. – 2013. – № 2 –
URL: www.science-sd.com/455-24426 (15.12.2025).









 PDF
PDF