About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Engineering
Introduction
A particular problem is now the encapsulation of drugs for use in medicine, pharmacology, providing safety, sustained release of drugs and biologically active substances from the capsules, taste-masking of bitter and nauseating drugs encapsulated supplements [1].
Currently, widespread drug packaging components gelatin - gelatin capsules. Capsules for drugs produce different shapes, sizes, and functional properties, which uses a special gelatin, which is available only by some enterprises in China and the European Union. The cost of this type of gelatin is in the range of 500-600 thousand rubles. per tonne and is increasing every year. This is due, first of all, the growing demand for the capsule, and secondly, the complexity and duration of the process of production of gelatin and growing needs of the product [2].
The annual volume of the Russian market of gelatin capsules is 1.2 billion pieces per year. The main producers of gelatin capsules, imported into Russia are Associated Capsules Pvt Ltd and Capsugel. Exports to Russia capsules or raw materials for their production in 2008 according to the newspaper "Kommersant - Siberia" was based on the finished product up to 1 billion units. year.
In this regard, there is now a problem of finding materials for production based on these capsules. A possible alternative manufacturing capsules are milk proteins. Presented in the form of various protein concentrates, milk proteins have a number of important functional and technological properties, which can also be changed purposefully. Availability of milk protein concentrates, and the possibility of widespread seasonal production of special technologies open up new possibilities of their use, especially in industries related to food technology (pharmaceuticals, medicine, biotechnology industry) [3].
¬ tional purpose of the thesis is to develop a research and technology capsules of milk proteins.
To achieve this goal the following objectives:
- Composition and properties of gelatin capsules;
- To evaluate the features of the composition and properties of milk protein concentrate in the use of technology in the capsules;
- To study the characteristics of hydrolysis of milk protein concentrate;
- Conduct research cleaning acid hydrolyzate of milk protein concentrate;
- To evaluate the organoleptic and physico-chemical properties and microbiological parameters obtained capsules;
- To release a test batch of industrial products, to assess the cost-effectiveness of its production.
Subjects and methods
The objects of study at various stages were [4]:
- Milk protein concentrate OST 10-02-02-3-87;
- DDS TU 6-09-64-75;
- Sodium benzoate SanPiN 2.3.2.1293-03
- Ethyl alcohol rectified GOST 18300-87
- Distilled water GOST 6709-72;
- Hydrochloric acid GOST 3118-78;
- Sulfuric acid GOST 45653-78;
- Sodium hydroxide GOST 4328-77;
- Raw materials and auxiliary materials that meet the requirements of the applicable documentation or received on imports and permitted for use Rospotrebnadzor (aromatic essences food OST 18-103 or aromatic composition).
The main raw materials and components of domestic production and imports produced to meet the requirements of SanPiN 2.3.2.1078. Minerals by microbiological must meet SanPiN 2.3.2.1078 (index 3.6.10.2).
Analyzed the organoleptic characteristics, physical-chemical and microbiological parameters.
Based on the implications of a standard documentation, conducted commercial adaptation of technology, defines the expected performance output.
When the work used standard, conventional and original research methods, including physical and chemical (thin-layer and gas chromatography, atomic absorption spectroscopy, Photocolorimeters, refractometers), biochemical and organoleptic and others [5].
Results and discussion
Given the objective analysis capsules organoleptic, physical, chemical, microbiological, defined fraction of the proteins gelatin mass.
In studies using the traditional technology to give a colorless transparent, clear or opaque monochrome, two-color transparent or opaque capsule, odorless, tasteless and have a smooth, homogeneous, without any visible damage and air and surface mechanical inclusions. Dissolution time capsule I in humans 12-15 minutes. and capsules II - 4-5 hours at 37 ± 2 º C, the mass fraction of water in the range 13-16%, the mass fraction of protein - 87,1-87,7 g/100 g capsules Kapsulometricheskie performance depends on the size of capsules. Weight capsules from 11.8 to 121.9 mg, the average occupancy 0,33-1,37 g capsules, the total length of the closed capsule 15,2-23,9 mm. Gelatinous mass composition represented protein fractions with a molecular weight 95,3-95,8 kDa.
In the future, these values ??are basic reference for the development of technology-based capsules of milk proteins (ICB).
Studied quality characteristics of milk proteins in the use of technology in food capsules. Conducted appropriate studies to assess the feasibility of using these proteins as a material for capsules.
As a result, interbank protein fractionation using a vertical electrophoresis identified the main faction of MBC, the estimation of their qualitative and quantitative composition. In Fig. 1 shows the
Detailed analysis of the data showed that about 39-40% IBC accounts for a s1-casein fraction, about 30-31% by a s2-and b-faction, though the last 6.9 times longer than a s2- caseins. Molecular weight fractions of casein varies from 20.8 to 25.8 kDa qualitative and quantitative composition of fractional MBC.
Qualitative and quantitative fractional composition according to IBC treatment results shown in Fig. 1 (B), is shown in Table. 1.
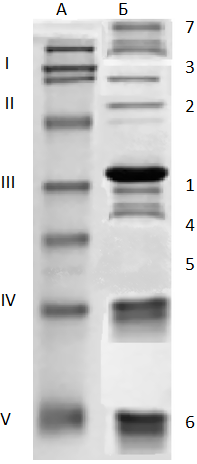
Table 1-Characteristics of the fractions of casein used IBC
|
Name faction |
Content,% |
Molecular weight, kDa |
|
αs1-casein |
39,6-40,1 |
23,4-25,5 |
|
αs2-casein |
3,8-4,3 |
24,9-25,8 |
|
β-casein |
27,6-28,1 |
23,7-25,2 |
|
χ-casein |
9,3-9,7 |
18,7-20,8 |
|
β-lactoglobulin |
8,7-9,2 |
17,6-18,8 |
|
α-lactalbumin |
3,6-4,1 |
14,2-15,1 |
|
Immunoglobulins |
3,1-3,6 |
398,7-409,4 |
|
Unidentified |
2,7-3,2 |
90,5- 95,9 |
The proportion of whey protein on total does not exceed 20.0%. Identified data demonstrate the use of IBC manufacture of capsules, but it would require a more quantitative approach to handling the fractional composition of the gelatin capsule weight.
The proteins in milk are the objects in the form of a complex structure of biopolymers. The most common of biopolymers are proteins mass 30-60 kDa. The spatial structure of the peptide chains of proteins found in milk objects are globular proteins. In turn, the polypeptide chain of the gelatin molecule has a spiral shape, which is located along a fixed chain intramolecular hydrogen bonds, refers to the fibrillar proteins. We believed that the partial destruction of the globular structure MBC, laying it in the form of linear polypeptides and further "merging" of polypeptides into fibrils give milk protein gelling properties of gelatin.
With the development of technology-based capsules MBC important issue of determining the optimal parameters targeted hydrolysis of milk proteins that maximize the deployment of a globular structure. Hydrolysis was carried out using 5 N acid and the qualitative and quantitative molecular weight distribution of the obtained fractions. Hydrolysis of the samples was carried out under vacuum (-0.8 MPa) at 110 ± 5 º C 2-8 pm Results of experiments on the effect of the duration of the process and the type of acid on the degree of hydrolysis of IBC are shown in Fig. 2.
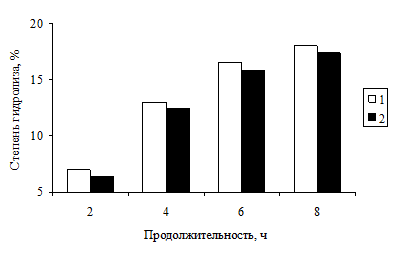
Fig. 2. The degree of hydrolysis of MBC depending on the length and type of acid: 1 - hydrochloric acid, 2 - sulfuric acid
Table 2 The relative content of fractions of casein MBC depending on the duration of acid hydrolysis name
|
Name faction |
The relative content of fractions of casein proteins, depending on the length of acid hydrolysis, h |
||||
|
0 |
2 |
4 |
6 |
8 |
|
|
a s1-faction |
39,4-40,8 |
36,5-36,9 |
34,0-34,6 |
30,5-31,9 |
26,6-27,8 |
|
a s2-fraction |
3,8-4,2 |
3,4-3,6 |
3,0-3,3 |
2,0-2,4 |
1,6-1,9 |
|
β-fraction |
26,9-28,3 |
25,5-25,8 |
23,7-24,1 |
19,3-20,6 |
17,1-18,5 |
|
g-fraction |
9,1-9,6 |
8,2-8,4 |
7,1-7,6 |
6,5-7,1 |
5,8-6,3 |
|
TOTAL |
80,0-82,1 |
73,6-74,7 |
67,8-69,6 |
58,3-62,0 |
51,1-54,5 |
|
β-lactoglobulin |
8,7-9,8 |
8,4-8,6 |
7,9-8,2 |
7,3-7,6 |
6,7-6,9 |
|
α-lactalbumin |
3,4-3,8 |
3,1-3,4 |
2,9-3,1 |
2,5-2,8 |
2,2-2,3 |
|
Immunoglobulins |
3,3-3,4 |
2,9-3,1 |
2,7-2,9 |
2,4-2,6 |
2,1-2,3 |
|
Unidentified |
2,5-3,0 |
2,3-2,5 |
2,0-2,3 |
2,2-2,3 |
2,0-2,1 |
|
TOTAL |
17,9-20 |
16,7-17,6 |
15,5-16,5 |
14,4-15,3 |
13,0-13,6 |
Further to analyze the duration of hydrolysis with hydrochloric acid, given the maximum accumulation of the main factions MBC, the most approximate molecular mass of fibrous proteins (Table 3).
Table 3 Molecular weight distribution of the hydrolyzate peptide fractions MBC depending on the duration of the process
|
Duration, h |
Relative molecular mass distribution, average molecular weight% |
Molecular weight MBC kDa |
||
|
более 56 кДа |
18-56 кДа |
менее 18 кДа |
||
|
0 |
73,0-73,0 |
23,0-27,0 |
0 |
52,6-56,3 |
|
2 |
70,0-74,0 |
26,0-30,0 |
0 |
45,7-46,9 |
|
4 |
64,0-68,0 |
15,0-19,0 |
13,0-21,0 |
34,1-36,9 |
|
6 |
39,0-43,0 |
31,0-35,0 |
22,0-30,0 |
15,8-18,6 |
|
8 |
31,0-35,0 |
42,0-46,0 |
19,0-27,0 |
12,2-14,3 |
The findings suggest that the minimum duration of hydrolysis MBC for two hours, in which the hydrolyzate ICB had an average molecular weight of more 45,7-46,9 kDa.
The results of the study determined ¬ Lena rational conditions for the hydrolysis of milk proteins with hydrochloric acid (temperature of 110 ± 5 º C, vacuum -0.8 MPa and duration of 120-125 minutes).
An analysis of published data cleaning process was carried out in two stages. In the first step, the removal of acid on a rotary evaporator under constant stirring and pressure -0.9 MPa. Results of the study on the correlation of temperature and duration of the process shown in Fig. 3.
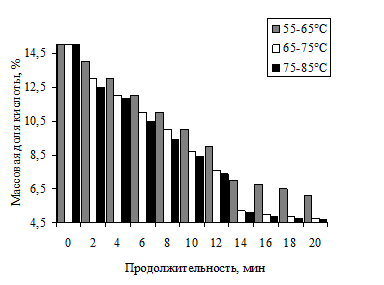
Fig. 3. Change in the mass fraction of acid on the temperature and duration of the process of self- processing MBC KG
Analysis of the effect of the temperature parameters showed that use temperature of 65 75 ° C most expedient but because at 55-65oS process is not intensive but, at 75 and 85 ° C, is energy wastage at the same rates acid removal.
At the second stage, the advanced treatment MBC KG on an ion exchange column to remove excess acid and other by organic substances. As the ion-exchange system, a mixture in equal parts nizkoosnovnyh macroporous anion type RELITE A330, RELITE A331.
Our study aims to determine the purity of the acid hydrolyzate (Fig. 4).
Table 4-The content of organic impurities before and after the ion exchange treatment
|
The value of organic |
Impurities, mg/100 g |
|
|
Before treatment |
After treatment |
|
|
Chloride |
3,4-3,5 |
0,2-0,3 |
|
Sulfates |
2,5-2,6 |
0,1-0,2 |
|
DDS |
2,1-2,3 |
0,0010-0,0015 |
Analysis of the results of experiments (Fig. 5 and Table. 4) revealed that, using the method of purification using ion exchange column may remove organic compounds for subsequent use in the food industry for the production of capsules.
The content of chloride ions is adequate. Sodium dodecyl sulfate (SDS) in the final product is substantially free. Further research is aimed at developing technologies for mass-based capsules KG MBC.

Fig. 4. Chromatograms of qualitative and quantitative content of organic impurities in the CG IBC: a, - before treatment, b, d - after treatment with 1 - chloride, 2 - sulfate, 3 – DDS
Based on the research results developed technological scheme of the capsules of milk proteins (Fig. 5).
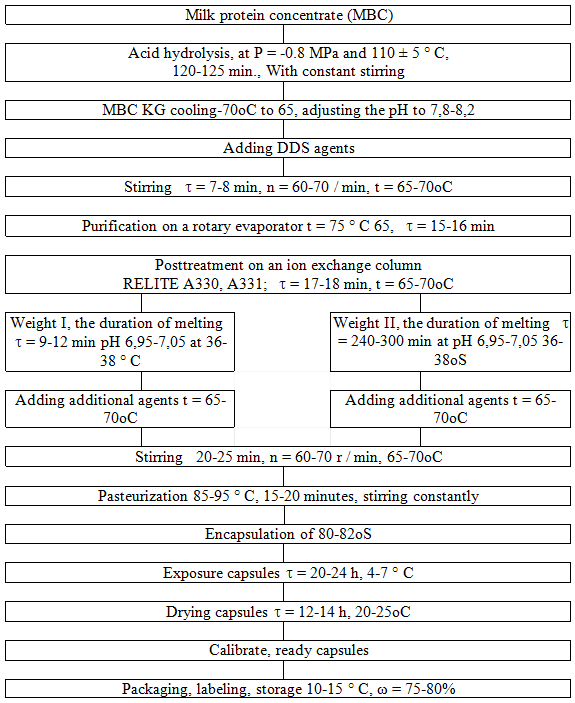
Fig. 5. Flow sheet of capsules based on milk proteins
A complex of chemical and physico-chemical studies to determine the processes occurring during the formation of capsules based on the acid hydrolyzate MBC. The quality of the capsules by MBC evaluated by organoleptic, physical, chemical and biological parameters (Table 5 - 8).
Table 5-Organoleptic capsules
|
Showing |
The correct form, with well-sealed and tightly capped, has a standard weight and size |
|
Consistency |
Dense vitreous structure, firm and restores consistency, not fragile and brittle with oversleeping and storage "in bulk", with oversleeping capsules with lids do not open last |
|
Taste and smell |
Typical of natural milk protein, if it is composed of fragrance and flavor, is typical of natural flavors and vkusozamenitelyu |
|
Color |
Corresponding to the natural color of milk protein, if the dye used, the peculiar form of dye. |
Table 6 Physical and chemical capsules MBC
|
Mass% |
Capsule I |
Capsule II |
|
Of dry matter, at least |
77,00±0,1 |
77,85±0,1 |
|
MBC, not less |
46,0±0,5 |
44,3±0,5 |
|
Plasticizer, no more than |
23,0±0,5 |
22,15±0,5 |
|
Additional agents, no more |
– |
3,7±0,5 |
|
stearic acid, not more than |
0,70±0,1 |
0,60±0,1 |
|
sodium benzoate, no more than |
0,10±0,1 |
0,10±0,1 |
|
sorbitol, no more than |
1,40±0,5 |
1,20±0,5 |
|
dyes, not more than |
0,05±0,1 |
0,05±0,1 |
|
flavors, no more |
0,05±0,1 |
0,05±0,1 |
Table 7 Weights and sizes of capsules based on MBK
|
Specifications |
Content Specifications |
|||||
|
Size |
00 |
0,l |
0 |
1 |
2 |
3 |
|
Weight capsules, mg |
109,2-121,9 |
97,5-116,5 |
89,3-104,5 |
69,9-81,8 |
56,1-65,9 |
45,2-11,8 |
|
Length of body capsule, mm |
19,8-20,6 |
20,9-22,63 |
16,9-18,7 |
14,8-16,8 |
14,6-15,9 |
12,8-14,6 |
|
Cover length capsule, mm |
11,3-12,9 |
12,1-13,9 |
10,3-11,3 |
9,5 -10,9 |
7,9-9,2 |
7,6-8,9 |
|
Total length of the closed capsule, mm |
22,9-23,9 |
22,2-23,8 |
21,0-21,9 |
18,1-19,3 |
17,4-18,9 |
15,2-16,8 |
|
Average capacity, g |
1,32-1,37 |
0,78-0,83 |
0,68-0,72 |
0,52-0,61 |
0,37-0,43 |
0,33-0,41 |
Table 8 Bioavailability capsules
|
Name the amino acid |
scale FAO / WHO, mg in 1 g of protein |
amino-acid score,% |
|
Valine |
50,0 |
112 |
|
Isoleucine |
40,0 |
95 |
|
Leucine |
70,0 |
100 |
|
Lysine |
55,0 |
96 |
|
Methionine + cystine |
35 |
98 |
|
Threonine |
40,0 |
117 |
|
Tryptophan |
10,0 |
103 |
|
Phenylalanine + Tyrosine |
60,0 |
128 |
A detailed analysis of the organoleptic, physical, chemical and microbiological properties as well as the amino acid composition of capsules of the technology showed that all indicators capsules based MBC comply SanPiN. The amino acid composition obtained capsules significantly higher than compared with gelatin capsules.
The calculated data show that the resulting product has a low cost compared to existing foreign counterparts and is affordable. We studied the cost characteristics of capsules based on protein hydrolysates. Found that the cost of producing capsules based MBC 2 times cheaper than the cost of gelatin capsules.
Table 9 Cost of raw material for the production of capsules I and II compared to gelatin, rub./1000 pc.
|
On the basis of ICB |
Based gelatin capsules |
||
|
Type I |
Type II |
Type I |
Type II |
|
36,57 |
36,26 |
67,45 |
71,37 |
Event was approved by the results of studies in industrial conditions at «issledovatelky Innovation Center."
Retsnzenty:
Nikolai Kucher, Doctor of Physical and Mathematical Sciences, Professor FGBOU VPO "KemSU";
Tatiana Shevchenko, Doctor of Technical Sciences, Professor FGBOU VPO "KemTIPP"
References
1. British Pharmacopoeia, 2000. – 2346 p176 European Pharmacopeia, 4th Ed. – Strasbourg: council of Europe, 2001. – 2416 p.
2. British Pharmacopoeia, 2000. – 2346 p.
3. Enciclopaedia of Pharmaceutical Technology / Ed. J. Swarbrick, I.C. Boylan. – 2-nd – New-York, Basel: Marcek Dekker, Inc., 2002. – Vol. 3. – 3032 p.
4. Guide to good Manufacturing Practice for medicinal Products/ The Rules Governing Medicinal Products in the European Community.– Vol.IV.–P.135.
5. Scientific Library dissertations disserCat http://www.dissercat.com/content/vliyanie-fiziko-khimicheskoi-modifikatsii-na-massoperenos-v-alginatnykh-gidrogelyakh#ixzz2IEJARKlR
Prosekov A.Ju., Ulrih E.V., Babich O.O., Ravnyushkin S.A., Drozdova T.M. THE USE OF ALTERNATIVE GELATIN FEEDSTOCK SHELLS SOFT CAPSULES. International Journal Of Applied And Fundamental Research. – 2013. – № 1 –
URL: www.science-sd.com/452-24050 (23.02.2026).









 PDF
PDF