About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
 PDF
p. 25-27
PDF
p. 25-27
The granular filters are distinguished from the following:
- by the highly complex refining capacity, not inferior to the CFF;
- by the filter elements production technology simplicity, in the form of the granules from the available and the inexpensive materials;
- by the unique and the unprecedented possibility to be varied by the filter composition, depending on the filter melt and the removed impurities nature;
- by the refining degree and the filter's capacity regulation controlling ability, at the expense of the bulked elements and the filter bed height size change, and the others.
The filter elements from the magnesite, in the form of the granules, with the 14-20 mm diameter, having received with the bonding materials and the agents quality application have been the research object: the sodium silicate aqueous solution and the hydrolyzed ethyl silicate ETHS - 40 (Table 1).
Table 1
The Materials' Characteristic, Having Used for the Filter Elements Preparation,
in the Form of the Granules
|
The Filter Element |
Bonding |
The Note |
||
|
Number |
The Granule Base |
The Outer Layer Material |
||
|
1 |
Magnesite |
MgO |
Sodium Silicate, ρ = 1,24 g/cm3 |
MgO melted |
|
2 |
Magnesite |
Y2O3 |
Hydrolyzed ethyl silicate ETHS-40 |
The Y2O3 layer on the magnesite granules has been obtained |
|
3 |
Magnesite with the aluminum powder |
MgO + Аldose |
Sodium Silicate, ρ = 1,24 g/cm3 |
The initial mixture composition 70 % MgO and 30 % Аl power [138] |
|
4 |
The fused alumina |
Аl2O3 |
SAG sulfite - alcohol grains |
The ceramic granules |
For the comparison, the ceramic granules from the fused alumina have been studied, having received by the solid - phase sintering (e.g. at 1,900 °С) under the industrial conditions, with the usage, as the SAG sulfite - alcohol grains bonding (e.g. option 4). So, the magnesite filter elements (e.g. option 1, 3) have been received by the roll briquetting method. The magnesite elements from Y2O3 (e.g. option 2) have been received, by means of the rolling (e.g. the cladding) of the deficient rare - earth oxide on the magnesite granules' surface. All these granules have not been exposed to the subsequent sintering just in these both cases (e.g. options 1-3).
Originally, the damp and the moist granules had been dried out in the air, after which they were placed into the drying cabinet, and immediately before filtering - they have been tempered and annealed at the ~900 °С temperature. In connection with above - mentioned material, the gravimetrical research on the weight change study of the filter elements have been carried out at the heating, originally, up to 200, and after that up to 900 °С. So, the studied granules in amount of 5 pieces from each option have preliminarily been weighed on the electronic balance, after that they have been laid on the special trays into the muffle furnace. So, the filter elements had been stood during an hour at this temperature, after the desired temperature reaching, after which the furnace was turned off. All these granules have been weighed again, having cooled down to the room temperature. Thus, the averaged data on the filter elements' mass change have already been presented in the Table 2. As it is seen from this Table, the granules' mass loss on the sodium silicate is in 5-6 times more, than at the ETHS-40 (e.g. options 1 and 2, correspondingly). This has been conditioned by the fact, that the sodium silicate is used, in the form of the aqueous solution, but the hydrolyzed ethyl silicate (ETHS) - is used with the organic solvent. So, the version 3 peculiarity and its special feature is the aluminum powder presence in the filter material. The high level dispersion of the last one is required the bonding solution increased consumption at the granulation. Therefore, all these filter granules mass losses at 200 °С almost in 2-3 times are exceeded the similar indicator for the options 1-2.
Table 2
The Filter Elements Mass Change at Their Heating
|
The Filter element (option №) |
The Mass Change, % mass |
|
|
at the granules heating temperature |
||
|
200 °С |
900 °С |
|
|
1 |
-1,40 |
-2,40 |
|
2 |
-0,33 |
-0,49 |
|
3 |
-2,63 |
+6,57 |
|
4 |
-0,85 |
-0,85 |
Note - the «+» and «-» signs are meant correspondingly the filter elements' mass «increase» or «decrease» at their heating.
But, at the 900 °С temperature, these granules mass, on the contrary, is being increased for 7-9 % relative to the initial one that is conditioned by the aluminum powder oxidation. So, the share of the last one, in the filter material structure, can be made up 10-30 %, so long as the Аl2O3 mass approximately in 2 times more the consumed mass for the aluminum its formation, than the mass increase, due to the aluminum powder oxidation, in the limit, is quite able to be equal to the mass share of the last one in the filter material. The ceramic granules (e.g. option 4) at their heating up to 200 °С are being lost their absorbed moisture, so their weight is left without any change at the subsequent heating.
Thus, the dilatometric measurements have been carried out at the installation, the diagram of which is given in the Fig. 1.
The installation had been mounted on the basis of the Tammann furnace 1 with the graphite heater. So, the studied filter granules 3 were laid on the bottom of the graphite shell 2, which was installed in the furnace on the support 4. The graphite rod 7 was lowered on the granule's top with the tip, in the form of the piston. Then, the weight 8, having had its mass 2 kg, was fixed on the rod. Then, the granules' heating was carried out in the inert atmosphere, for what the argon was laid up from below. The thermocouple 6 and the potentiometer 5 were used for the tests' temperature measurement. So, the BP5/20 thermocouple junction was placed in the special hole in the bottom of the graphite shell 2. The tests' maximum temperature was made up 1,580 - 1,590 °С. The granules layer initial height was made up 45 mm. The granules layer height change was fixed, by means of the registering device 9, having transformed the rod 7 linear motions into the electrical signals, on the device 10. The measurements were carried out accurate to ±1 mm. The filter height change at the heating in the graphic form has been shown in the Fig. 2.
So, the linear dimensions increase is quite characterized for all the filter elements versions in the initial stages of the heating, due to the thermal expansion. The elements, having manufactured with using, as the bonding sodium silicate (e.g. the curve 1), have the extreme dependence of the linear dimensions change from the temperature; so, it is obvious, that the granules expansion is being stopped, and their shrink is being started at the 1,370 °С temperature. So, the sodium silicate change for the hydrolyzed ethyl silicate (ETHS-40) is prevented the granules softening and their shrinkage (e.g. the curve 2). The most linear dimensions change is characterized for the filter element from the sintered fused alumina (e.g. the curve 4). The filter element from the magnesite with the aluminum powder mixture has the characteristics, which are quite close to it (e.g. the curve 3). That is why, the last presence is prevented the filter element softening, in spite of the use, as the sodium silicate bonding.
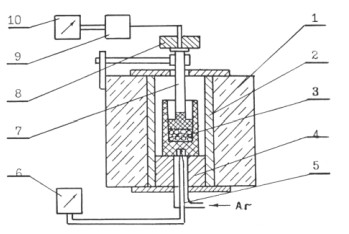
Fig. 1. The installation diagram for the filter elements dilatometric measurements at the heating:
1 - furnace; 2 - shell; 3 - filter elements (e.g. granules); 4 - support; 5 - thermocouple;
6, 10 - potentiometers; 7 - rod; 8 - weight; 9 - transformer
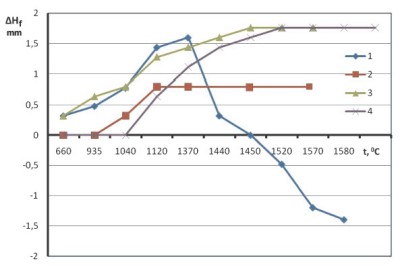
Fig. 2. The filter height change at the heating:
1 - MgO (on the sodium silicate); 2 - MgO (on the ethyl silicate);
3 - MgO + Al por. (on the sodium silicate); 4 - Al2O3 (ceramic)
Thus, the gravimetric and the dilatometric already performed researches and the studies have been shown, that the studied bonding materials use is allowed to be received the filter elements just without the high - temperature solid - phase sintering. So, it is quite necessary to be entered not less 5 % aluminum powder into the fireproof and the refractory material, at the melt large masses filtration, for the purpose of the filter elements softening prevention, having manufactured with the use, as the sodium silicate bonding material.
Kimanov B.M., Ten A.B., Isagulov A.Z., Zholdubaeva Z.D. THE GRAVIMETRIC AND DILATOMETRIC RESEARCH OF THE THREE - DIMENSIONAL FILTER ELEMENTS BEHAVIOUR AT HEATING. International Journal Of Applied And Fundamental Research. – 2012. – № 1 –
URL: www.science-sd.com/450-24007 (15.02.2026).