About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
The therapeutic potential of interleukin (IL-2) to cure breast cancer (BC) is still not clear both in mice [1] and human patients [2] although distinct therapeutic benefit has been achieved in limited cohorts of patients [3]. We hypothesized that a therapeutic IL-2 potential might be revealed when evaluated for short and long survivors separately, as both benefit and non-benefit subgroups exist in IL-2 treated mice with transplanted mammary carcinoma (MC) [4]. Earlier we have shown promising anti-cancer potential of a single IL-2 treatment in various transplanted MC models for the only recipients with early emerging MC [4, 5]. Moreover, we have found prognostic factors among 30 laboratory hematological and biochemical parameters that predicted early manifestation of transplanted MC [6].
The aim of this paper was to (1) to measure 17 routine hematological parameters in intact males before mammary cancer transplantation, (2) to apply low dose IL-2 therapy twice only to recipients with early appearing tumors, and (3) to disclose differences in initial hematological parameters for short and long control and treated survivors.
MATERIALS AND METHODS
Mice
Mice of BLRB- Rb(8.17)1Iem (BLRB) strain with high incidence of naturally arisen mammary carcinoma (MC) were maintained in non-SPF thoroughly controlled conditions at Mouse Breeding Facility at the Department of biotechnology, Institute of Bioorganic Chemistry, RAS, Moscow [1]. Mice were fed according Institutional guidance and author´s supplementation and got water ad libitum. Each mouse had individual mark and was followed through the whole lifespan as a veterinary patient.
Blood sampling
At day 0 blood samples (0.3-0.5 ml per male) were collected from the retroorbital vein sinus of intact BLRB males under ether narcosis (n=64) two weeks before MC cell inoculation.
The individual values of the following seventeen hematological parameters were measured using the hematological analyzer "MICROS OT 18" (Roche, Switzerland): RBC - red blood cell count, 1012/L; HCT- hematocrite, %; HGB - hemoglobin, g/L; MCV - mean corpuscular volume, fl; MCH - mean corpuscular hemoglobin, pg; MCHC - mean corpuscular hemoglobin concentration, g/L; RDW - red blood cells distribution width, %; PLT - platelet count, 109/L; PCT - thrombocrite, %; MPV - median platelet volume, fl; PDW - platelets distribution width, %; WBC - white blood cell count, 109/L; LY - lymphocytes, %; MO - monocytes, %; EO - eosinophils, %; SN- segmented neutrophils, %; BN - band neutrophils, %. Leukocytes were distinguished by routine microscopic methods.
Experimental design
At day 14 after blood sample collection, 107 mammary carcinoma cells from a syngeneic BLRB spontaneous mammary carcinoma were transplanted s.c. into right fat pads near the axillaries of 64 BLRB males according to [6]. At day 13 after MC inoculation 13 males aged 12.1+0.7 months (weight 26.5+0.5g) with early emerging MCs of 5.8+0.3 mm in size were selected. Eight males with tumors of 6.3+0.5 mm in mean diameter were treated at day 13 and day 22 by peritumoral injection of 2.5 x 105 U Chiron IL-2 (thereafter called low dose) suspended in 0.5 ml containing 0.9% NaCl and 0.1% Bovine Serum Albumin (BSA). Control mice (n=11) with tumors of 5.4+0.4 mm in mean diameter were injected in the same manner with 0.5 ml 0.9% NaCl/0.1% BSA. Mice were inspected each day for survival and health monitoring, and once a week for tumor size measurement. Tumor growth rate parameters, namely, mean tumor diameter (TD) and relative tumor diameter (RTD) as a measure of tumor growth increase in relation to its initial size before the therapy start were calculated according to [5]. The significance of differences in averages was determined by the parametric Student´s t-test. Results are presented as means + SEM.
RESULTS
1. Therapeutic effect of two low peritumoral IL-2 doses (Figure 1)
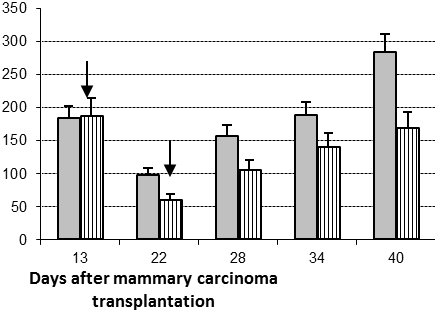
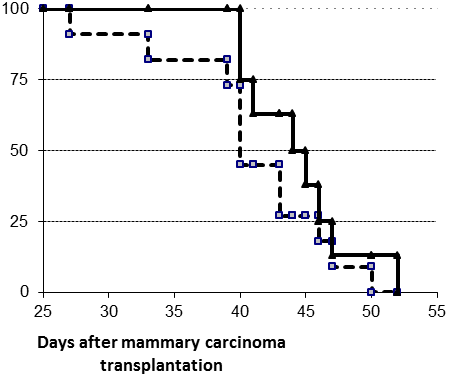
Therapeutic efficacy of two low IL-2 doses was tested in the only males with early emerging MCs. Eight males were treated twice with a peritumoral IL-2 treatment (2.5x105 IU per mouse per injection) at days 13 and 22 after MC cell inoculation; eleven non-IL-2 treated males constituted the control group. The average tumor diameter dynamics in control and treated groups did not differ significantly (data not shown). However, the relative tumor diameter (RTD) was significantly smaller in the IL-2 treated group (Figure 1A, p<0.05) showing that used therapy modality caused on average inhibition of tumor growth in treated group. The survival of the whole IL-2 treated group was improved only slightly compared with the survival of controls. However, 100% of the treated animals were alive at day 40 post tumor transplantation (ptt) versus only 73% in control group (Figure 1B). The survival curves for both the IL-2 treated and control mice showed two distinct periods, namely before and after day 41 ptt. This was the reason for further search of blood parameters that can predict short term (death within 41 ptt) and long term (death after day 41) survival in treated and control mice.
А |
B |
Figure 1. Relative tumor diameter and survival of the whole IL-2 treated and control groups. Two peritumoral 2.5x105 IL-2 treatments (arrows) were applied to early emerging mammary carcinomas at day 13 and day 22 post tumor transplantation. Average relative tumor diameter (RTD, A) and survival (B) for the whole control (grey columns and cubes, respectively) and IL-2 treated (striped columns and triangles, respectively) groups.
2. Tumor growth in short and long survivors (Figure 2)
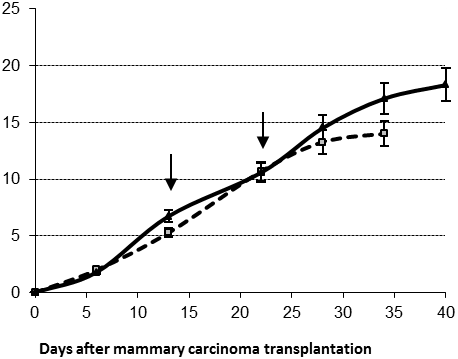
The average survival time for short survivors in control and IL-2 treated groups did not differ significantly (37±2 and 40±0.3 days, respectively). However, short survivors comprised only 3/8=37% of IL-2 treated group versus 6/11=54% of the control group. Two locoregional injections of IL-2 (2x105 IU per mouse per application) at days 13 and 22 resulted in significant tumor diameter increase (Figure 2A, day 34 ptt, p<0.05).
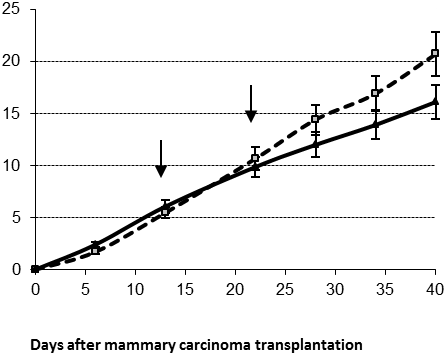
The average survival time for long survivors in control and treated groups was similar, namely 46±1 and 47±1 days, respectively. However, the proportion of long survivors in IL-2 treated group was higher than in the control one (63% versus 46%, respectively). For these long survivors the same IL-2 therapy mode resulted in a significant tumor growth delay (Figure 2B, day 40 ptt, p<0.05).
A |
B |
Figure 2. Average tumor diameter in control (cubes) and treated (triangles) males for short (A) and long (B) survivors after two IL-2 applications (arrows); *- p<0.05
No differences were observed in the average age and blood sampling for short and long survivors. Lung, spleen, and kidney weight tended to be elevated in long survivors compared with short survivors, and in IL-2 treated mice compared with controls (data not shown). Only liver weight was significantly increased in the IL-2 treated long survivors (1450±103 mg) compared with control long survivors (1110±106 mg, p<0.05). Then we compared hematological parameters within short and long survival subgroups measured in all mice prospectively before mammary carcinoma transplantation in an attempt to predict short or long survival of tumor-bearing mice after IL-2 treatment.
Prognostic factors among 17 hematological parameters measured in intact males before MC inoculation
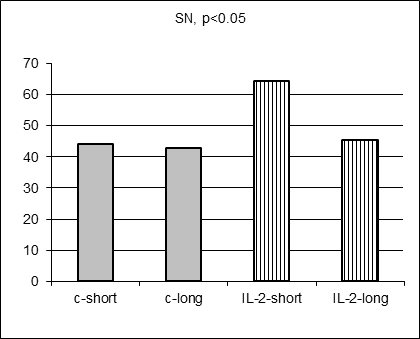
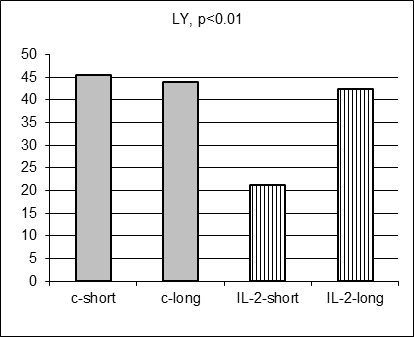
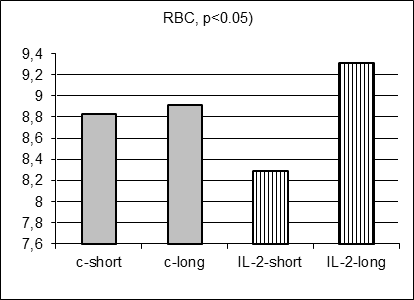
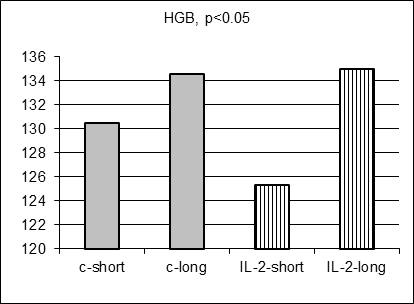
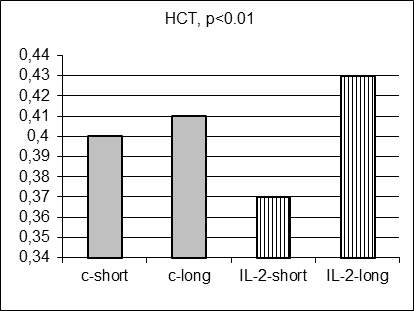
As anticipated, there were no statistically significant differences in 17 hematological parameter values measured in control and IL-2-treated males prospectively before MC inoculation and IL-2 treatment (data not shown). Moreover, there were no statistically significant differences in average hematological parameter values measured in control short and control long survivors (including Figure 3 A-E, grey columns). However, surprisingly 5 hematological parameter values (SN, Ly, RBC, HGB, and HST) differed significantly for short and long survivors within IL-2 treated group (Figure 3A-E, stripped columns). Both control and IL-2-treated short survivors exhibited noticeably higher leukocytosis than long survivors of both subgroups (data not shown). However, the proportion of segmented neutrophils was in average significantly elevated in IL-2 treated short survivors comparing with treated long survivors (Figure 3A, left and right stripped columns, respectively, p<0.05) being at the similar average level in both short and long control survivors. Furthermore, the proportion of lymphocytes was on average significantly decreased in blood of IL-2 treated short survivors compared with the proportion in treated long survivors (Figure 3B, left and right stripped columns, respectively, p<0.01) being at the similar average level in both short and long control survivors. The average amount of RBC in blood of IL-2 treated short survivors was diminished comparing with the level of RBC in blood of treated long survivors (Figure 3C, left and right stripped columns, respectively, p<0.05) being at the similar average level in both short and long control survivors. Average HGB and HCT levels were diminished in males that survived shortly after MC inoculation and two IL-2 applications (Figure 3D and 3E, p<0.05 and p<0.01, respectively) although control short survivors demonstrated the similar tendency.
|
|
|
|
|
|
|
Figure 3. Hematological parameters for control (grey columns) and IL-2 treated (striped columns) short (left) and long (right) survivors. Significant differences in the parameter values were observed only between short and long treated survivors |
These data pointed to the frequent chronic inflammation, anemia and leucopenia in the IL-2-treated short survivors; whereas non-treated short survivors did not exhibit these disorders significantly more often than control long survivors.
DISCUSSION
Early and late emerging transplanted mouse tumors are always found in recipient mice receiving mammary carcinoma cells from the same donor tumor [5-7]. Previously, we found that (1) some routine blood and serum biochemical parameters do predict early tumor manifestation and (2) only recipients bearing early appeared mammary carcinomas survived better after a single peritumoral IL-2 application two weeks ptt. Therefore, we were interested whether some blood parameters measured prospectively in intact mice can predict IL-2 therapy efficacy in a mouse model of breast cancer? To this end, 17 routine clinical hematological parameters were measured in 64 intact BLRB males 2 weeks before MC inoculation. Two weeks after tumor cell inoculation an early emerging tumor subgroup (19 males) was distinguished. Eight males were treated with IL-2, whereas 11 males bearing tumors of the same average size of about 5mm appearing at the same time constituted the control group. No significant differences in both tumor growth rate and survival were found in the IL-2-treated males compared with controls.
Analyzing individual data we proposed that only for distinct recipients IL-2 therapy was beneficial, being non-beneficial for the rest. Therefore, short and long survivors were distinguished in both control and IL-2-treated groups using the same criterion, namely death before and after day 41 ptt, respectively. We looked for a possible connection between blood laboratory parameters measured in naive mice and further long or short survival time of tumor-bearing males with or without IL-2 therapy. As anticipated, we found no differences in average initial laboratory parameter values between IL-2-treated and non-treated groups. Moreover, there were no significant differences in all hematological parameter values between short and long survivors in control groups. Surprisingly, we do found significant differences in 5 hematological parameter levels among short and long survivors of the IL-2 treated group.
This analysis shows that a few males with leucopenia and anemia before mammary carcinoma transplantation survived significantly shorter after tumor cell inoculation and IL-2 therapy than healthy animals from treated group. Opposing to the inclined fall of poor condition mice to the short survivor treated subgroup, in non-treated group recipients with poor physical conditions dispersed accidentally to short or long survivors. This interpretation shows that weak physical condition of murine mammary carcinoma recipients might serve as a limitation of IL-2 therapeutical efficacy (at least, for the application mode used). These data are in agreement with the recommendation of Kedar and Klein to select only human cancer patients with good physical condition for immunotherapeutical regimens (e.g., with a Karnofsky performance status of more than 70%) as patients with severe leucopenia, cardiovascular, respiratory, renal or liver disorders should not receive certain types of treatment [8].
Interestingly, in the untreated group the probability to survive shorter or longer after MC inoculation seems to be more or less similar for males with and without hematological disorders as all prognostic parameter values had the similar average level (SN, LY, RBC) or were only slightly different (HGB, HST)) in control-short and control-long survivors.
CONCLUSIONS
Taken together, these data (1) demonstrated that both benefit (long survivors) and non-benefit (short survivors) murine mammary carcinoma recipients exist after two low dose peritumoral IL-2 applications and (2) demonstrated prognostic value of five hematological parameters to predict the advantage of IL-2 therapy mode used.
REFERENCES
- Moiseyeva E.: Anti-breast cancer drug testing. Original Approaches. Novel Set of Mouse Models. Lambert Ac. Publ., (2009), p.p. 220
- Grande C., Firvida J.L., Navas V., Casal J. (2006) Anticancer Drugs, 17, 1-12
- Nicolini A., Carpi A. (2009) Med Res Rev, 29, 436-471
- Moiseyeva E.V., Semushina S.G., Chaadaeva A.V., Kessler Yu.V. (2010) Int J Appl Fundam Res, 10, in publication
- Moiseyeva E.V., Merkulova I.B., Bijleveld C., Koten J.W., Miroshnikov A.I., Den Otter W. (2003) Cancer Immunol Immunother, 52, 487-496
- Moiseyeva E.V., Bojenko V.K., Fomina G.G., Krasnovskaya O.R., Krasilshchikova M.S., Den Otter W. (2002) Baltic J Lab Anim Sci, 12, 74-83
- Moiseyeva E.V., Rapoport E.M., Bovin N.V., Miroshnikov A.I., Chaadaeva A.V., Krasilshchikova M.S., Bojenko V.K., Bijleveld C., van Dijk J.E., Den Otter W. (2005) Breast Cancer Res Treat, 91, 227-241
- Kedar E., Klein E. (1992) Adv Cancer Res, 59, 245-322
E. Moiseyeva, S. Semushina, Yu. Kessler, E. Skrabelinskaya and V. Bojenko SEVERAL BLOOD PARAMETERS IN INTACT MICE CONNECTED WITH THEIR LONG SURVIVAL AFTER MAMMARY CANCER TRANSPLANTATION AND INTERLEUKIN-2 TREATMENT. International Journal Of Applied And Fundamental Research. – 2010. – № 4 –
URL: www.science-sd.com/386-23435 (03.10.2025).









 PDF
PDF