About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Numerous studies presented evidence that breast cancer (BC) patients exhibit T -cell mediated functional immunosuppression, which progresses during tumor growth, so that even the early localized disease shows distinct defects [1]. And high-grade generalized immune dysfunction in advanced disease is characteristic for either tumor-bearing mice [2] or BC patients [3, 4]. Moreover, BC adjuvant therapy (chemotherapy, radiotherapy, or their combination) significantly delayed immune restoration in numerous immune parameters, but especially in interleukin-2 (IL-2) level recovery [5]. Local IL-2 deficiency was demonstrated within breast carcinoma [6]; and IL-2 augmented tumoricidal function of each leukocyte population within tumor infiltrated leucocytes [7]. Thus, wide broad evidence was accumulated more than three decades ago for the in vivo IL-2 therapeutic administration to BC patients. Data obtained in experimental research provided rationale to apply IL-2 locally [8].
IL-2 belongs to mediators that are produced by T cells and exert multiple, pleiotropic effects in an autocrine or paracrine fashion [9]. Specific activities of IL-2 as a member of γC cytokine family are nowadays well documented in both natural and therapeutic settings [10]. The stimulation of a tumour-specific T-cell response has several theoretical advantages over other forms of cancer treatment. Namely, T cells can home to antigen-expressing tumor deposits and continue to proliferate in response to immunogenic proteins expressed on cancer cells until all the tumour cells are eradicated. Moreover, immunological memory can be generated, allowing for eradication of antigen-bearing tumors if they reoccur [11].
The IL-2 therapy alone and/or in combinations with other immunotherapeutical modalities is currently a distinct immunotherapy mode in BC clinic (discussed in [11, 12]) while it´s therapeutic potential is still not clear both in mice [13] and human patients [14]. However, clear therapeutic benefit has been achieved in limited cohorts of patients [3, 15]. Indeed, from either theoretical point of view (due to dual nature of this cytokine) or applied implementations (numerous lessons from animal research) one may expect differing IL-2 effects on tumor growth parameters as IL-2 plays quite opposite roles for the T-effector and T-regulatory arms of the immune system (discussed in [16, 17]).
Using IL-2 to enhance T-cell immunity one should keep in mind crucial importance of application time during immune response in vitro. At the time of initial T-cell activation IL-2 increased the size of the CD8+ memory pool but reduced the parameter if present during memory maintenance by inhibiting the proliferation of CD8+ memory cells. Thus, IL-2-based immunotherapeutical strategies should take into account the divergent roles of IL-2 in CD8+ T cell immunity [18].
As early as 1969, T-cell mediated local IL-2 therapy was shown to be sensitive to the start and duration time of IL-2 administration in several animal tumor models in vivo [19]. Timing of IL-2 administration and T-cell differentiation status were investigated in details in EL-4 tymoma mouse model [20]. Administration of IL-2 during the initial phase of the response, clonal expansion, and development of effector function, had no effect on the number of cytotoxic T-lymphocytes (CTL) generated or the control of tumor growth. In contrast, a short 2-day time course of low-dose IL-2 at the peak of clonal expansion or at later times resulted in prolonged and expanded responses by the CTL, with concomitant decrease in tumor load and extension of survival. However, when IL-2 administration was more prolonged, as is often the case in clinical trials, the therapeutic benefit was lost due to elimination of the tumor-specific CTL, at least in part through induction of apoptosis [20].
Previously we have shown that on average anti-cancer IL-2 effect may be hardly seen in several transplantable mouse models of BC when tumor growth and survival parameters are estimated for the whole treated and control groups [21]. We showed that distinct therapeutic effect for the whole IL-2 treated group might be hidden as both benefit and non-benefit subgroups exist within treated tumor-bearing mice. Therefore, to reveal masked IL-2 potency tumor parameters should be analyzed separately for short and long survivor, for instance. Using similar subgrouping approach, retrospective research of Characiejus et al. demonstrated that immunotherapy by interferon increased overall survival only in the subgroup of renal cell carcinoma patients who had shorter survival potency, whereas for patients with longer survival potency immunotherapy obviously tended to shorten the survival [22].
Keeping in mind the theoretical importance of timing schedule in the IL-2 immunotherapy protocol and going back to applied implementation of this point in a given mouse model we question whether the time of IL-2 application itself or the initial size of an individual tumor at the therapy start is of a particular value to predict recipient outcome. To address this query specifically we develop mouse model of BC in BLRB males with the first transplantation generation from spontaneous female mammary adenocarcinoma (MAC). We applied IL-2 locally only ones for the tumors of 5 mm in size 2 weeks post transplantation (p.t.) for the first subgroup of mice with early emerging MAC (short subclinical period, subshort), and 7 weeks p.t. for the second subgroup with lately emerging MAC (long subclinical period, sublong).
MATERIALS AND METHODS
Mice
We used mice of our inbred strain BLRB- Rb(8.17)1Iem (thereafter called BLRB) with high incidence of naturally arisen mammary adenocarcinoma (MAC) [13]. Animals were maintained in non-SPF thoroughly controlled conditions.
Experimental design
Twenty-six relatively old BLRB males (~12 months of age) were used for MAC cell inoculation to mimic cancer appearing in elderly. Tumor cells were taken from two naturally arisen fast and slowly growing syngeneic female MACs. At day 0, 107 cells from this suspension were injected in male mice subcutaneously near right fad pads. At day 14 seven males with early emerging subshort tumors of about 5 mm in diameter were treated peritumorally with 2.5 x 106 U Chiron IL-2 suspended in 0.5 ml containing 0.9% NaCl and 0.1% Bovine Serum Albumin (BSA). Six control mice with tumors of the similar size were injected in the same manner with 0.5 ml 0.9% NaCl/0.1% BSA (vehicle) at the same time. Six males with late emerging sublong tumors of about 5 mm in diameter were treated by IL-2 in the same manner at day 51; seven control males with the tumors of the same size received vehicle. Mice were inspected each day for survival and health monitoring. The mean tumor diameter was measured once a week as described in [13].
Statistical Analysis
The Mann-Whitney non-parametric U-test was used to compare tumor growth kinetics and survival dynamics.
RESULTS
No differences in average tumor diameter were found after a single IL-2 application at day 14 p.t. to subshort MACs versus controls. However, the IL-2 treated males survived significantly longer (p<0.05, fig. 1, white figures).
No differences in average tumor diameter were seen after IL-2 application at day 51 p.t. to sublong MACs versus controls; while IL-2 treated males survived shorter than the controls. The survival dynamics differed significantly from day 35 until day 135 (p<0.05, fig.1, black figures).
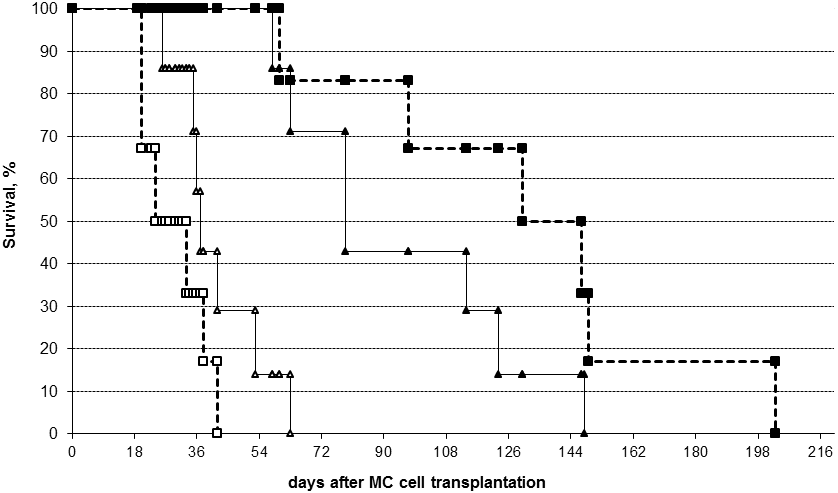
Figure 1. Survival dynamics of IL-2 treated (triangles) and control (cubes, dotted lines) mice with early (white figures) and late (black figures) emerging mammary adenocarcinomas (MAC). Early emerging MACs with short subclinical period (subshort, white triangles) were treated with a single peritumoral IL-2 injection at day 14. Late emerging MACs with long subclinical period (sublong, black triangles) were treated at day 51 after tumor cell inoculation. Controls with subshort tumors (white cubes) and sublong tumors (black cubes) were treated with a vehicle at the same time as corresponding IL-2 treated mice.
DISCUSSION
Early and late emerging transplanted mouse MACs were demonstrated in recipient mice suggesting distinct similarity to human BC where both aggressive and indolent forms occur [23]. Obtained data showed that single peritumoral IL-2 application against early emerging mouse MAC near the second week p.t. resulted in significant survival improvement in the BLRB mouse model, similarly to previously published data in A/Sn and BALB/c transplantable MC with the long transplantation history [21]. However, mice that acquired tumors of the same initial size considerably later and were treated at 8th week p.t. survived shorter than controls.
These data show clearly that in the model used duration of subclinical period was of particular importance rather then the initial tumor size. This seems to be in discordance with [24], where the benefit of serial intratumoral IL-2 therapy depended on initial low tumor burden and failed in large mesotheliomas. However, tumor burden is a function of post transplantation time. Thus, similarly to our research, in [24] anti-cancer IL-2 therapy also failed being applied too late.
Kiessling et al. revealed different effects of fast and slowly growing transplanted murine cancer on the host immune system causing local versus generalized tumor-associated immune suppression, respectively [2]. From this point of view IL-2 immunotherapy might fail in mice bearing late emerging, slowly growing MACs, at least partly due to generalized tumor-associated immunodeficiency.
Furthermore, recently published data support IL-2 essential role in immune tolerance rather than early paradigm in which IL-2 was a central for protective immune system [16]. From one side, based on the IL-2 effect on T-effector arm both IL-2 and it´s Russian analog roncoleukin are currently used to augment anticancer response, i.e. boost autoimmune reaction against autologous tumor [25]. Based on the IL-2 effect on T-regulator arm both pharmaceutical forms of cytokine are used to down regulate response in autoimmune patients [17]. Therefore, not only some parts of these newly published data remain controversial, but even cytokine itself paradoxically proposed to augment and suppress generally the same process, i.e. immune response to self-antigens.
We assume that these two sides of the IL-2 effect can be distinguished in vivo in tumor-bearing host by means of different application time of cytokine. Probably, during early time (~2weeks p.t.) IL-2 application may contribute to protected T-effector arm immunity causing survival benefit. However, being applied too late (~7week p.t.) IL-2 may actively supply another arms of it´s activity, namely apoptosis of T-effectors and development of memory T-cells and/or T-regulators (Treg), which were shown to suppress effector reactions [16, 17].
Developing subgrouping approach, we disclosed promising anti-cancer potential of a single IL-2 treatment in various types of transplanted BC after therapy efficacy estimation in early and late emerging MAC subsets separately [21]. Survival improvement was found only for recipients bearing tumors with short subclinical periods; whereas survival of animals bearing MACs with long subclinical periods was shortened.
These findings from mouse models of BC may be translated to clinic. Although initial tumor size at BC diagnosis is one of the most used among other prognostic factors in clinic [23] to predict patient´s outcome [26]; it may be essential but not sufficient to forecast benefit of the IL-2 immunotherapy. Duration of subclinical period may be of significant independent importance; although this parameter value in BC clinic is hardly estimated as most tumors are several years old at initial presentation [27].
CONCLUSIONS
Taken together, obtained data (1) demonstrate both benefit and non-benefit effects of IL-2 therapy on recipient survival in mouse model of BC used and (2) compel to search for prognostic factors that may predict the therapeutic effect of IL-2 therapy. These findings may facilitate to develop basic principles of a selection procedure for BC patients who may benefit from local IL-2 therapy as was proposed by Kedar and Klein almost three decades ago [28].
REFERENCES
- Blidaru A., Bordea C.I., Viisoreanu C.G., Bordea M., Iliescu I., Dutescu D., Radu F., Dragoescu H. (1998) Rom J Physiol, l 35, 127-134
- Kiessling R., Wasserman K., Horiguchi S., Kono K., Sjoberg J., Pisa P., Petersson M. (1999) Cancer Immunol Immunother, 48, 353-362
- Hadden J.W. (1999) Int J Immunopharmacol, 21, 79-101
- Pockaj B.A., Basu G.D., Pathangey L.B., Gray R.J., Hernandez J.L., Gendler S.J., Mukherjee P. (2004) Ann Surg Oncol, 11, 328-339
- Kang D.H., Weaver M.T., Park N.J., Smith B., McArdle T., Carpenter J. (2009) Nurs Res, 58, 105-114
- Venetsanakos E., Beckman I., Bradley J., Skinner J.M. (1997) Br J Cancer, 5, 1826-1830
- Wong P.Y., Staren E.D., Tereshkova N., Braun D.P. (1998) J Surg Res, 76, 95-103
- Bernsen M.R., Tang J.-W., Everse L.A., Koten J.-W., Den Otter W. (1999) Cancer Treat Rev, 25, 73-82
- Kroemer G., Toribio M.L., Martinez C. (1991) New Biol, 3, 219-229
- Overwijk W.W., Schluns K.S. (2009) Clin Immunol, 132, 153-165
- Disis M.L., Bernhard H., Jaffee E.M. (2009) Lancet, 373, 673-683
- Nicolini A., Carpi A. (2009) Med Res Rev, 29, 436-471
- Moiseyeva E (2005) Utrecht University, The Netherlands 191 pp, http://igitur-archive.library.uu.nl/dissertations/2005-1130-200033/index.htm
- Grande C., Firvida J.L., Navas V., Casal J. (2006) Anticancer Drugs, 17, 1-12
- Nicolini A., Carpi A., Rossi G. (2005) J Immunother, 28, 276-279
- Malek T.R. (2008) Annu Rev Immunol, 26, 453-479
- Montero E., Alonso L., Perez R., Lage A. (2007) Ann N Y Acad Sci, 1107, 239-250
- Dai Z., Konieczny B.T., Lakkis F.G. (2000) J Immunol, 165, 3031-3036
- Everse L.A., Bernsen M.R., Dullens H.F., Den Otter W. (1996) J Exp Ther Oncol 1, 231-236
- Shrikant P., Mescher M.F. (2002) J Immunol, 169, 1753 - 1759
- Moiseeva E.V., Merkulova I.B., Bijleveld C., Koten J.W., Miroshnikov A.I., Den Otter W. (2003) Cancer Immunol Immunother, 52, 487-496
- Characiejus D., Pasukoniene V., Kazlauskaite N., Valuckas K.P., Petraitis T., Mauricas M., Den Otter W. (2002) Anticancer Res, 22, 3679-3683
- Harris J.R., Morrow M., Bonadonna G. (1993) In.: Cancer Principl Pract Oncol 1264-1332
- Jackaman C., Bundell C.S., Kinnear B.F., Smith A.M., Filion P., van Hagen D., Robinson B.W., Nelson D.J. (2003) J Immunol, 171, 5051-5063
- Molchanov O., Karelin M., Zharinov G. (2002) Cytokin Inflam, 1, 38-47
- Burke H.B. (2004) J Natl Cancer Inst, 96, 1408-1409
- Friberg S., Mattson S. (1997) J Surg Oncol, 65, 284-297
- Kedar E., Klein E. (1992) Adv Cancer Res, 59, 245-322
E. Moiseyeva, S. Semushina, A. Chaadaeva and Ju. Kessler THEORETICAL AND APPLIED IMPLICATIONS OF DUAL INTERLEUKIN-2 NATURE: SINGLE LOCAL APPLICATION IN A MOUSE BREAST CANCER MODEL. International Journal Of Applied And Fundamental Research. – 2010. – № 4 –
URL: www.science-sd.com/386-23434 (29.06.2025).









 PDF
PDF